Preparation and adsorption properties of fluorine-doped porous polymers
-
摘要: 共轭微孔聚合物(CMPs)由于其稳定的孔隙结构、多样的合成方法及在气体吸附、废水染料吸附等领域的应用受到了广泛关注。设计并合成了两种氟掺杂共轭微孔聚合物:首先合成了两种含氟构筑单元1,3,5-三氟-2,4,6-三乙炔基苯(FAB)和5,10,15,20-四(2-氟-4-乙炔基苯基)卟啉(TFPP)。分别以FAB和TFPP为构筑单元,以1,1’-二溴二茂铁(Fc(Br)2)为链接单元,基于Sonogashira偶联反应,成功制备了两种氟掺杂多孔聚合物氟掺杂多孔聚合物二茂铁基(Fc)-FAB-CMP 和 Fc-TFPP-CMP。并进一步对两种多孔聚合物的化学结构、热稳定性、孔隙结构、气体及染料吸附性能等方面进行了详细的分析。结果表明,两种聚合物均表现出良好的热稳定性。Fc-FAB-CMP和 Fc-TFPP-CMP的BET比表面积分别为302.89 m2/g和125.2 m2/g,其中Fc-FAB-CMP表现出更加优异的气体吸附性能。此外,二茂铁单元与氟取代基的引入可成为阳离子染料甲基紫(MV)的吸附位点,Fc-FAB-CMP相较于许多更高比表面积的聚合物表现出对MV更优异的吸附能力,最大吸附量可达318 mg/g。Abstract: Conjugated microporous polymers (CMPs) have attracted extensive attention due to their stable pore structure, various synthesis methods and applications in gas adsorption and dye adsorption in wastewater treatment. Based on the Sonogashira coupling reaction, two fluorine-doped porous polymers (synthetic route of ferrocene (Fc)-1,3,5-trifluoro-2,4,6-triethynylbenzene (FAB)-CMP, Fc-5,10,15,20-tetrakis(2-fluoro-4-ethynylphenyl) porphyrin (TFPP)-CMP) were designed and synthesized by using FAB and TFPP as the center construction units, 1,1'-dibromoferrocene (Fc(Br)2) as a linking unit. The chemical structure, thermal stability, elemental composition and particle morphology of CMPs were investigated in detail. The results show that the fluorine-doped porous polymer is successfully synthesised by the Sonogashira coupling reaction, and the product exhibits good thermal stabi-lity and stable porosity. The BET specific surface areas of Fc-FAB-CMP and Fc-TFPP-CMP are 302.89 m2/g and 125.2 m2/g, respectively. Moreover, the introduction of ferrocene and fluorine can be the adsorption site of cationic dye methyl violet (MV), the highest adsorption value of Fc-FAB-CMP for MV reaches 318 mg/g.
-
Key words:
- porous /
- fluorine-doped /
- gas adsorption /
- dye adsorption /
- ferrocene
-
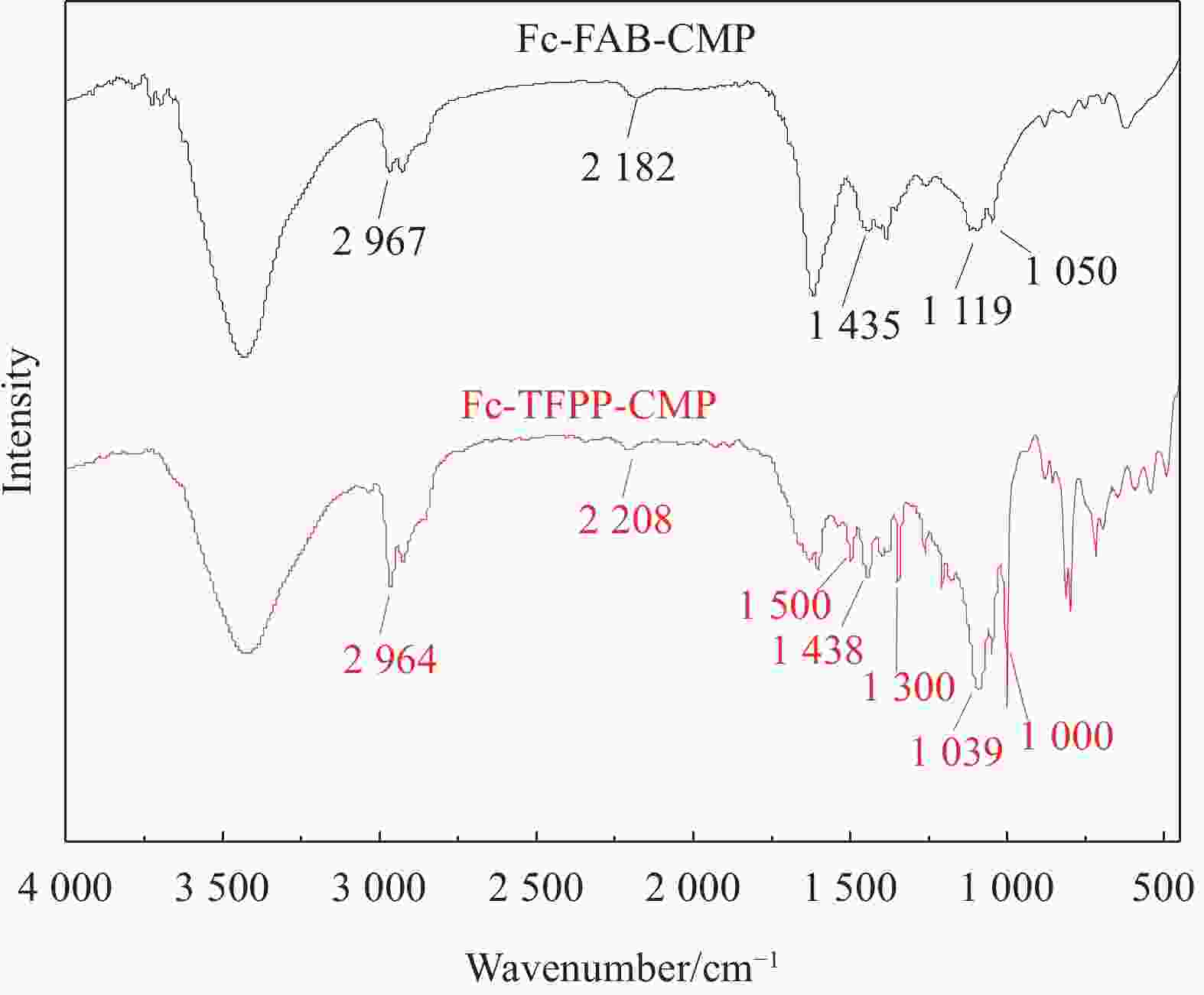
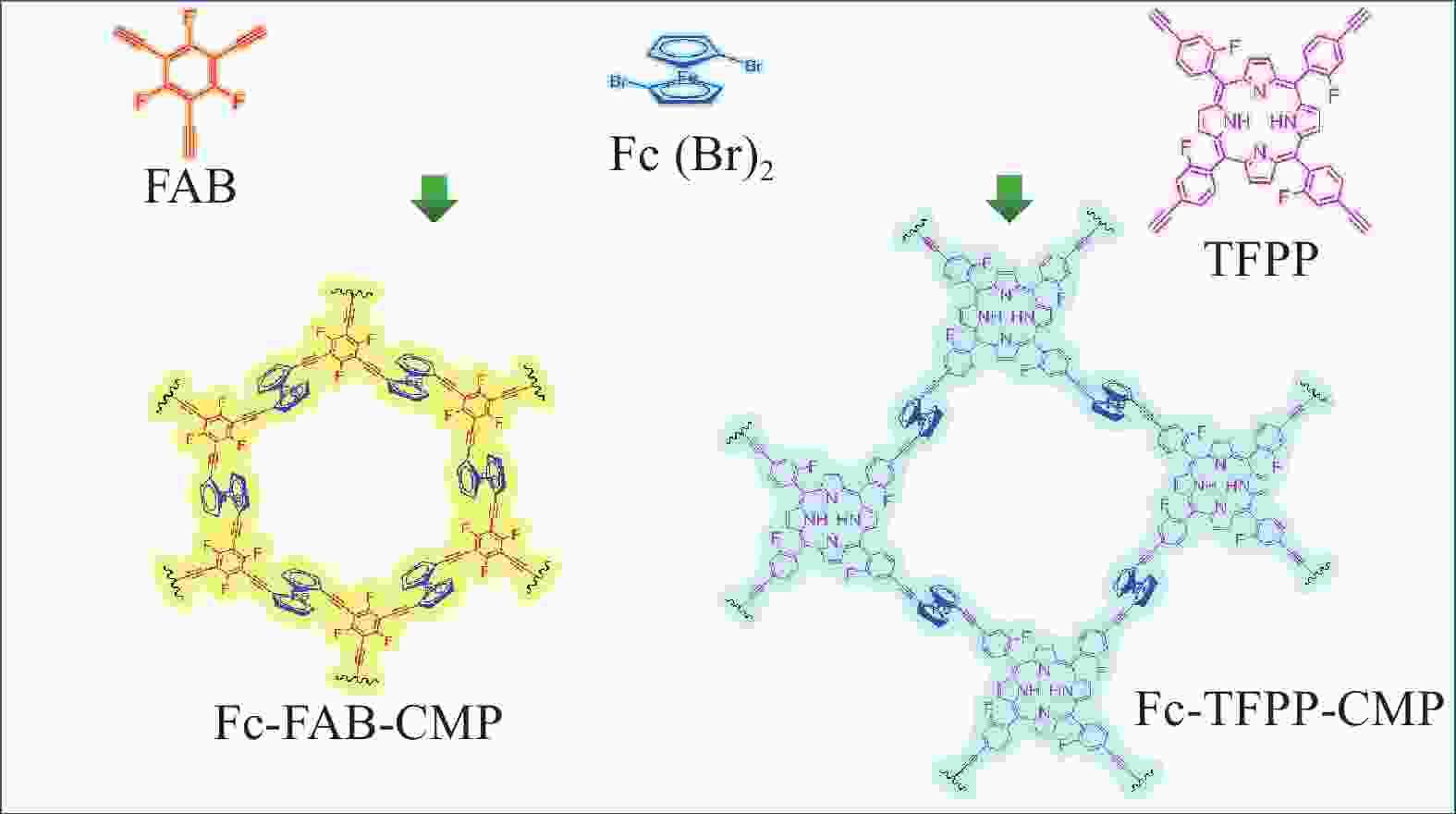
图 1 氟掺杂多孔聚合物二茂铁基(Fc)-1,3,5-三氟-2,4,6-三乙炔基苯(FAB)-共轭微孔聚合物(CMP)和Fc-5,10,15,20-四(2-氟-4-乙炔基苯基)卟啉(TFPP)-CMP制备路线图
Figure 1. Synthetic routes of ferrocene (Fc)-1,3,5-trifluoro-2,4,6-triethynylbenzene (FAB)-conjugated microporous polymers (CMP) and Fc-5,10,15,20-tetrakis(2-fluoro-4-ethynylphenyl) porphyrin (TFPP)-CMP
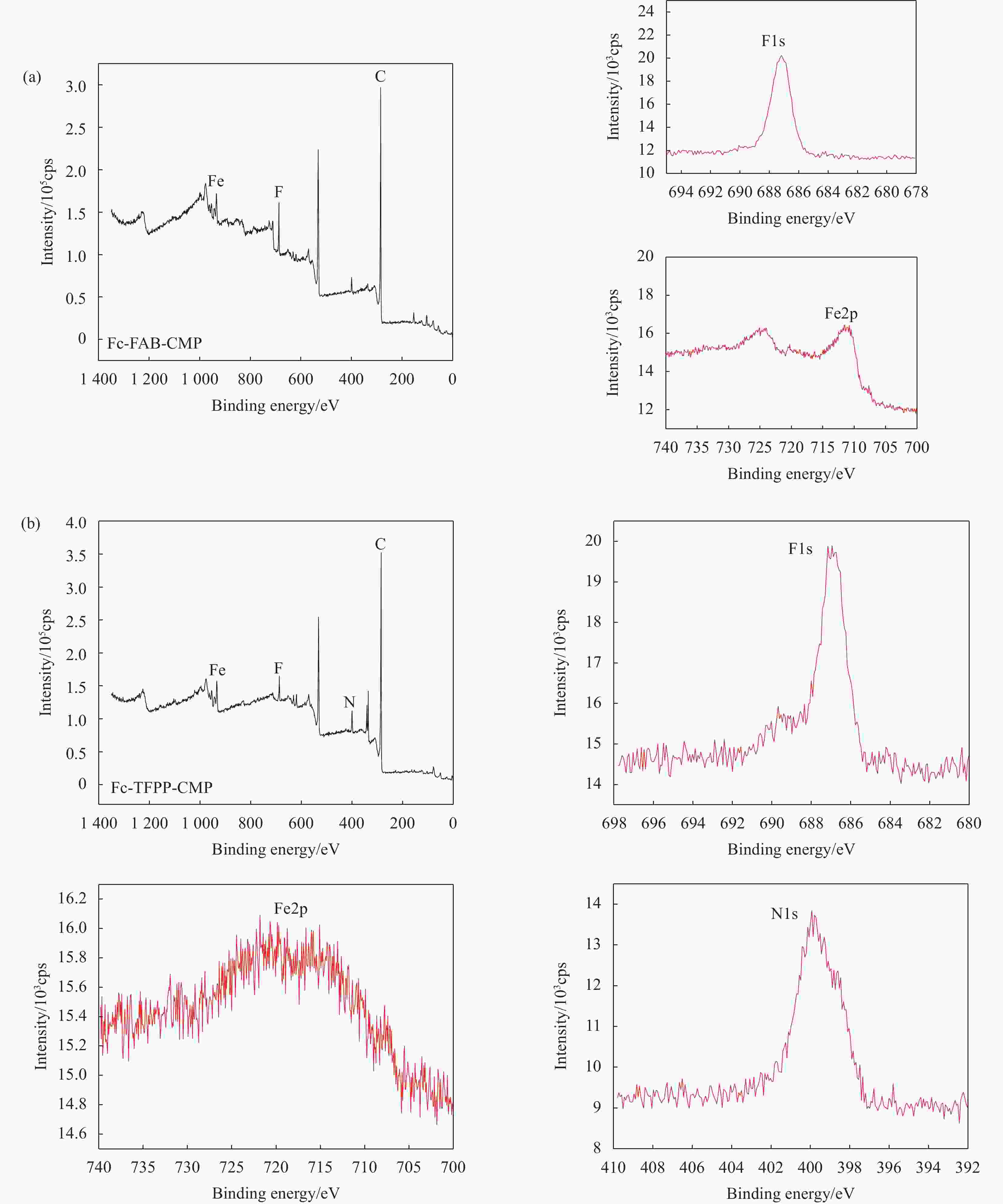
表 1 Fc-FAB-CMP和Fc-TFPP-CMP元素含量(at%)
Table 1. Element contents of Fc-FAB-CMP and Fc-TFPP-CMP (at%)
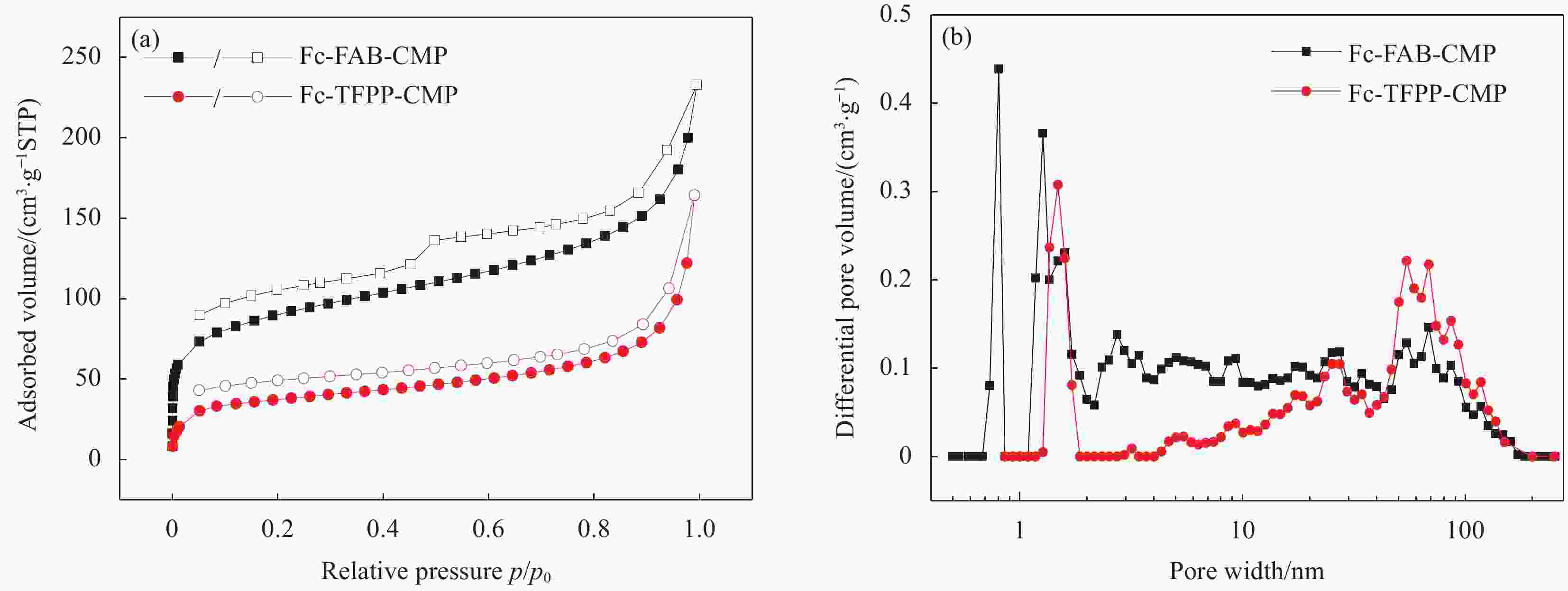
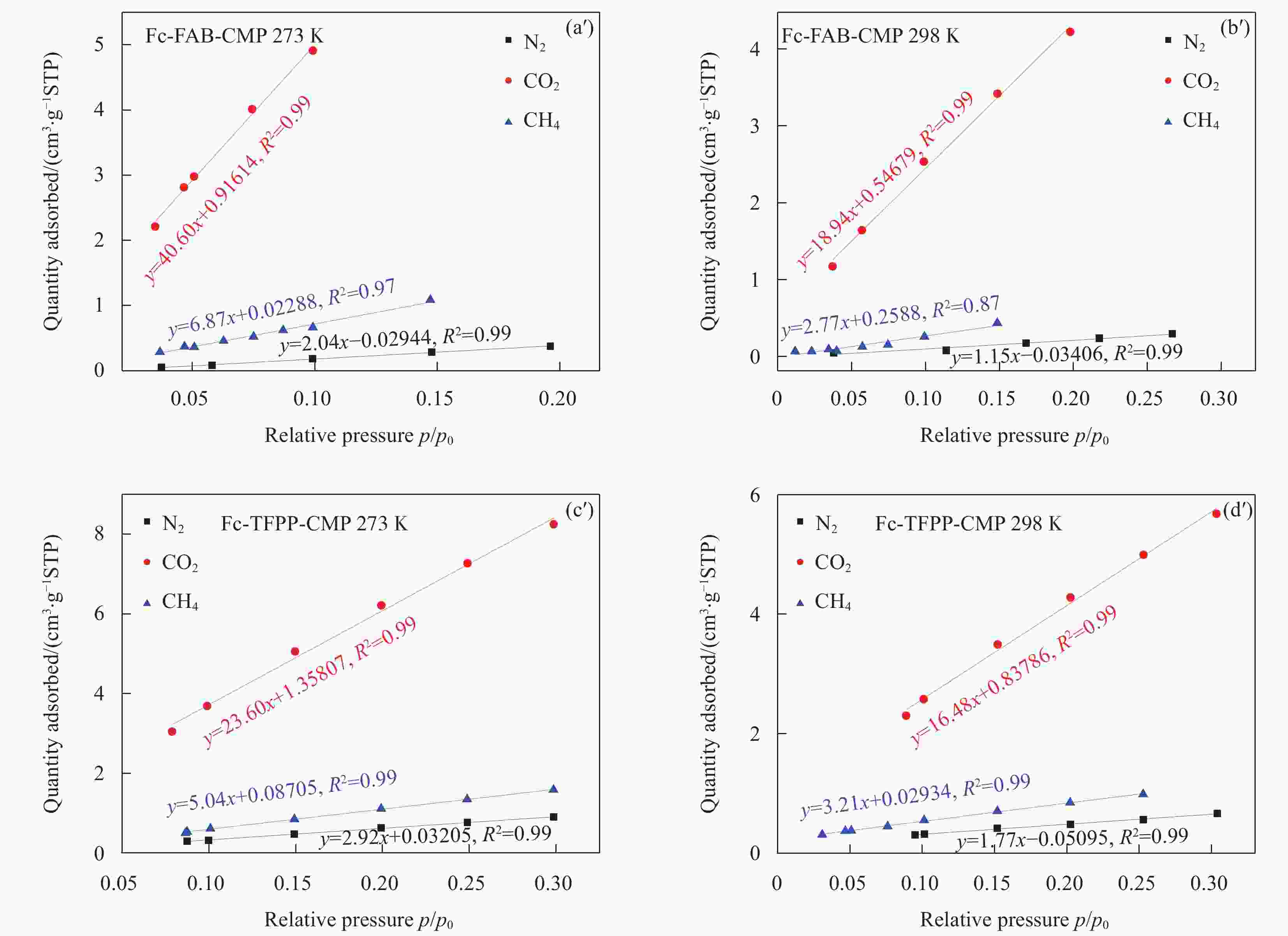
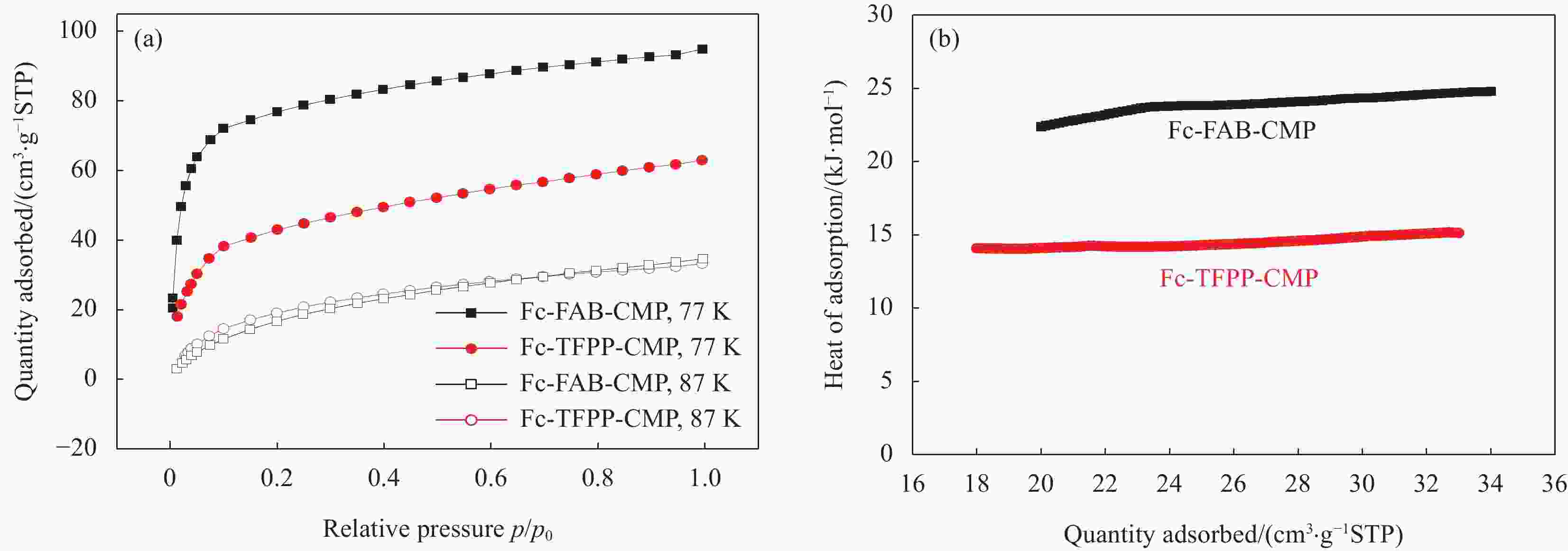
Material C Fe F N Fc-FAB-CMP 89.66 4.26 6.08 — Fc-TFPP-CMP 87.88 1.28 3.59 7.26 表 2 Fc-FAB-CMP和Fc-TFPP-CMP的多孔性质
Table 2. Porosity data of Fc-FAB-CMP and Fc-TFPP-CMP
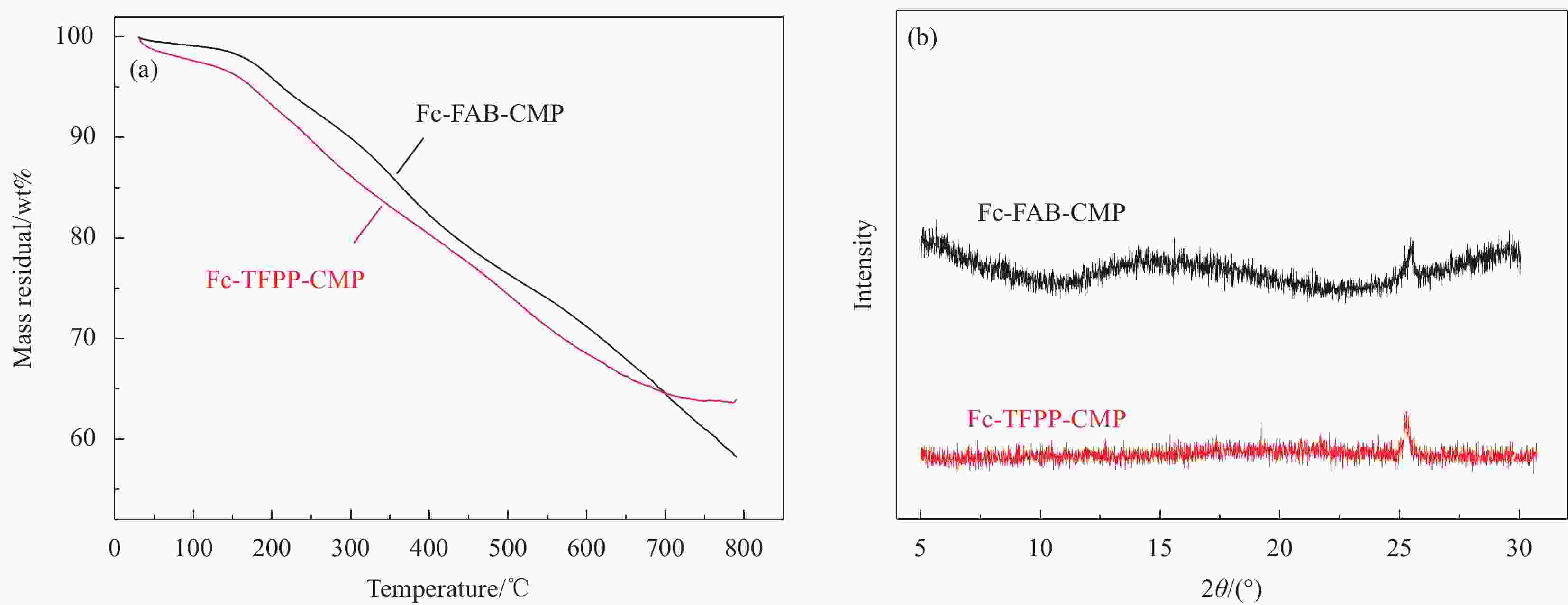
Sample SBET
/(m2·g−1)Smicro
/(m2·g−1)Smicro/SBET Vtotal
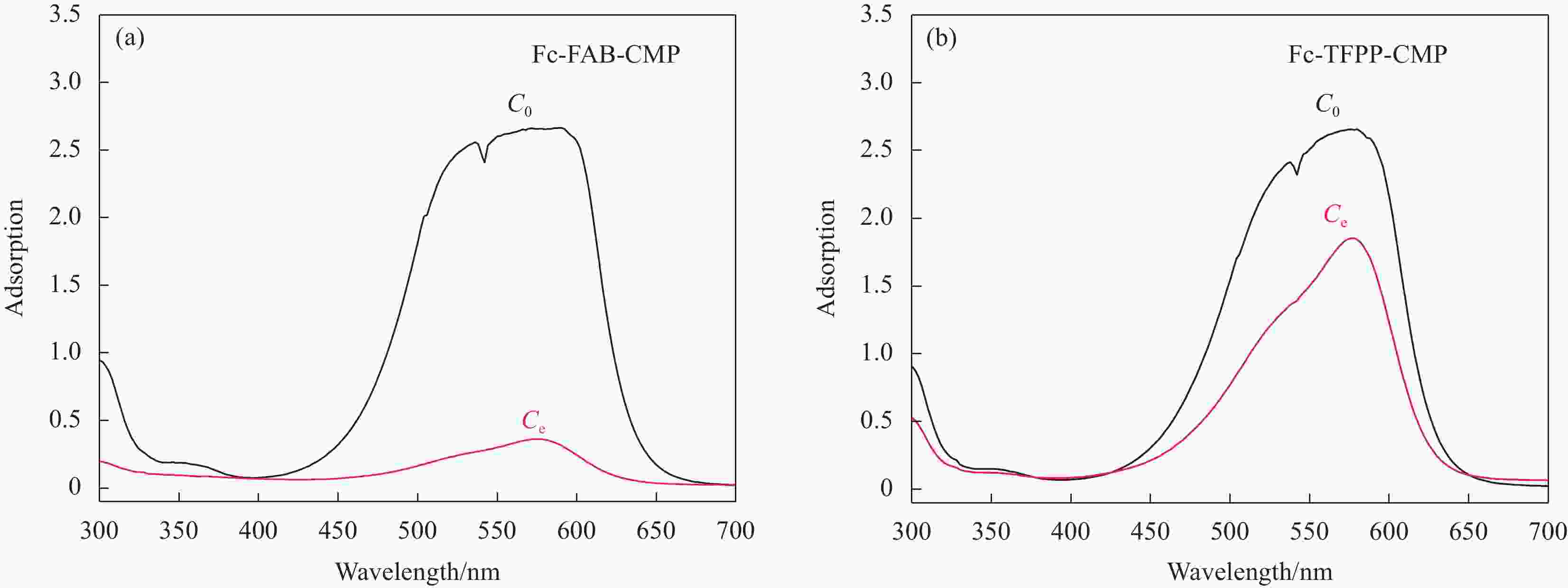
/(cm3·g−1)Fc-FAB-CMP 302.9 106.8 0.35 0.27 Fc-TFPP-CMP 125.2 47.1 0.38 0.14 Notes: SBET—Specific surface area calculated from the nitrogen adsorption isotherm using the BET method; Smicro—Micropore surface area calculated from the nitrogen adsorption isotherm using the t-plot method; Vtotal—Total pore volume. 表 3 多种有机多孔材料常温、常压条件下对MV的吸附能力对比
Table 3. Comparison on adsorption capacity of organic porous materials to MV
Sample SBET/(m2·g−1) Mmax/(mg·g−1) Reference Fc-FAB-CMP 303 318 This work Fc-TFPP-CMP 125 120 This work ASRM 130 61 [43] Cu(BDC-NH2)(4,4’-Bipy)(0.5) 124 60 [44] CMK-3 1568 158 [45] Notes: Mmax—Maximum adsorption of MV; ASRM—Activated sintering process red mud; Cu(BDC-NH2)(4,4’-Bipy)(0.5)—An anionic metal-organic framework; CMK-3—A mesoporous carbon material. 表 4 Fc-FAB-CMP和Fc-TFPP-CMP对MV的动力学参数和相关系数R2
Table 4. Kinetic parameters and correlation coefficient R2 for MV of Fc-FAB-CMP and Fc-TFPP-CMP
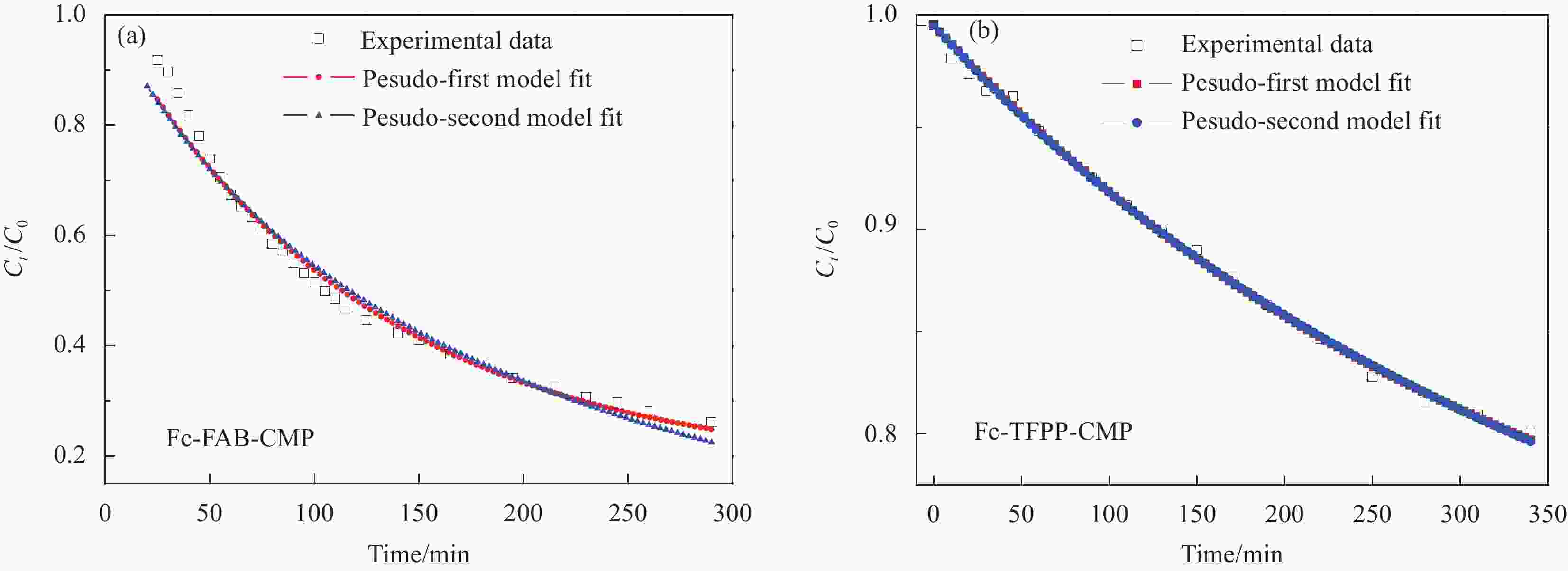
Sample Model Qe
/(mg·g−1)k1/k2 R2 Fc-FAB-CMP First 265.28 4.08×10−3 0.966 Second 393.53 7.46×10−6 0.978 Fc-TFPP-CMP First 115.86 2.92×10−3 0.996 Second 193.03 9.34×10−6 0.996 Notes: Qe—Adsorption capacity at equilibrium; k1 and k2—Adsorption rate constants of the pesudo-firs model and the pesudo-second model, respectively; R2—Correlation coefficient. -
[1] 张雪杨, 王挺, 吴礼光, 等. 介孔碳/聚酰亚胺杂化膜原位聚合法制备及其气体分离性能[J]. 复合材料学报, 2018, 35(11):2958-2965.ZHANG X Y, WANG T, WU L G, et al. Fabrication of mesoporous carbon/polyimide hybrid membrane by in-situ polymerization and their gas separation performance[J]. Acta Materiae Compositae Sinica,2018,35(11):2958-2965(in Chinese). [2] KUPGAN G, ABBOTT L J, HART K E, et al. Modeling amorphous microporous polymers for CO2 capture and separations[J]. Chemical Reviews,2018,118(11):5488-5538. doi: 10.1021/acs.chemrev.7b00691 [3] AHMED D S, EL-HITI G A, YOUSIF E, et al. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review[J]. Journal of Polymer Research,2018,25(3):1-21. [4] 杨金辉, 胡世琴, 杨斌, 等. 氨化烟末生物碳吸附剂的制备及其对Cr(VI)的吸附行为[J]. 复合材料学报, 2022, 39(1):236-245.YANG J H, HU S Q, YANG B, et al. Preparation of ammoniated tobacco leave powder residue biochar and its adsorption behavior on Cr(VI)[J]. Acta Materiae Compositae Sinica,2022,39(1):236-245(in Chinese). [5] 费佳颖, 梁艺萱, 李寒冰, 等. 离子液体改性金属-有机骨架复合材料的构筑策略及在环境介质中的应用[J]. 复合材料学报, 2022, 39(6):2527-2542.FEI J Y, LIANG Y X, LI H B, et al. Construction strategy of ionic liquid modified metal-organic framework composite and application in environmental medium[J]. Acta Materiae Compositae Sinica,2022,39(6):2527-2542(in Chinese). [6] ALTAVA B, BURGUETE M I, GARCIA-VERDUGO E, et al. Chiral catalysts immobilized on achiral polymers: Effect of the polymer support on the performance of the catalyst[J]. Chemical Society Reviews,2018,47(8):2722-2771. doi: 10.1039/C7CS00734E [7] WU J, XU F, LI S, et al. Porous polymers as multifunctional material platforms toward task-specific applications[J]. Advanced Materials,2019,31(4):1802922. doi: 10.1002/adma.201802922 [8] 武鑫霞, 曹占平, 苏婷, 等. Ce改性金属有机骨架材料对氟的吸附[J]. 复合材料学报, 2020, 37(10):2636-2644.WU X X, CAO Z P, SU T, et al. Adsorption of Ce modified metal organic framework to fluorine[J]. Acta Materiae Compositae Sinica,2020,37(10):2636-2644(in Chinese). [9] LATROCHE M, SURBLÉ S, SERRE C, et al. Hydrogen storage in the giant-pore metal-organic frameworks MIL-100 and MIL-101[J]. Angewandte Chemie, International Edition,2006,45(48):8227-8231. doi: 10.1002/anie.200600105 [10] ZHENG S T, YANG H Z Y. Designed synthesis of POM-organic frameworks from [Ni(6)PW(9)] building blocks under hydrothermal conditions[J]. Angewandte Chemie,2008,47(21):3909-3913. doi: 10.1002/anie.200705709 [11] SUN D, SIMMONS J M, COLLIER C D, et al. Metal-organic framework from an anthracene derivative containing nanoscopic cages exhibiting high methane uptake[J]. Journal of the American Chemical Society,2008,130(3):1012-1016. doi: 10.1021/ja0771639 [12] LIU Q Q, XIA B J, HUANG J, et al. Hypercrosslinked polystyrene microspheres with ultrahigh surface area and their application in gas storage[J]. Materials Chemistry and Physics,2017,199:616-622. doi: 10.1016/j.matchemphys.2017.07.032 [13] URBAN C, MCCORD E F, WEBSTER O W, et al. 129Xe NMR studies of hyper-cross-linked polyarylcarbinols: Rigid versus flexible structures[J]. Chemistry of Materials,1995,7(7):1325-1332. doi: 10.1021/cm00055a008 [14] NÚRIA F, MANESIOTIS P, SHERRINGTON D C, et al. Synthesis of spherical ultra-high-surface-area monodisperse amphipathic polymer sponges in the low-micrometer size range[J]. Advanced Materials,2008,20(7):1298-1302. doi: 10.1002/adma.200702237 [15] EL-KADERI H M, HUNT J R, MENDOZA-CORTES J L, et al. Designed synthesis of 3D covalent organic frameworks[J]. Science,2007,316(5822):268-272. doi: 10.1126/science.1139915 [16] CÔTÉ A P, BENIN A I, OCKWIG N W, et al. Porous, crystalline, covalent organic frameworks[J]. Science,2005,310(5751):1166-1170. doi: 10.1126/science.1120411 [17] CÔTÉ A P, EL-KADERI H M, FURUKAWA H, et al. Reticular synthesis of microporous and mesoporous 2D covalent organic frameworks[J]. Journal of the American Chemical Society,2007,129(43):12914-12915. doi: 10.1021/ja0751781 [18] DOGRU M, SONNAUER A, GAVRYUSHIN A, et al. A covalent organic framework with 4 nm open pores[J]. Chemi-cal Communications,2011,47(6):1707-1709. doi: 10.1039/c0cc03792c [19] COOPER A I. Conjugated microporous polymers[J]. Advanced Materials,2009,21(12):1291-1295. doi: 10.1002/adma.200801971 [20] CAO X X, WANG R Y, PENG Q, et al. Effect of pore structure on the adsorption capacities to different sizes of adsorbates by ferrocene-based conjugated microporous polymers[J]. Polymer,2021,233:124192. doi: 10.1016/j.polymer.2021.124192 [21] KIM H, CHA M C, PARK H W, et al. Preparation of a Yb (III)-Incorporated porous polymer by post-Coordination: Enhancement of gas adsorption and catalytic activity[J]. Journal of Polymer Science Part A: Polymer Chemistry,2013,51(24):5291-5297. doi: 10.1002/pola.26962 [22] LIU Q Q, LI G, TANG Z, et al. Design and synthesis of conjugated polymers of tunable pore size distribution[J]. Materials Chemistry and Physics,2017,186:11-18. doi: 10.1016/j.matchemphys.2016.06.004 [23] JIANG J X, SU F, TREWIN A, et al. Conjugated microporous poly(aryleneethynylene) networks[J]. Angewandte Chemie, International Edition,2010,119(45):8728-8732. [24] RITCHIE L K, TREWIN A, REGUERA-GALAN A, et al. Synthesis of COF-5 using microwave irradiation and conventional solvothermal routes[J]. Microporous and Mesoporous Materials,2010,132:132-136. doi: 10.1016/j.micromeso.2010.02.010 [25] 吴可义, 郭佳. 多尺度共轭微孔聚合物的可控合成[J]. 化学学报, 2015, 73:480-486. doi: 10.6023/A15020138WU K Y, GUO J. Controllable synthesis of multi-scale conjugated microporous polymer[J]. Acta Chimica Sinca,2015,73:480-486(in Chinese). doi: 10.6023/A15020138 [26] XU Y H, JIN S B, XU H, et al. Conjugated microporous polymers: Design, synthesis and application[J]. Chemical Society Reviews,2013,42:8012-8031. doi: 10.1039/c3cs60160a [27] KISKAN B, WEBER J. Versatile postmodification of conju-gated microporous polymers using thiolyne chemistry[J]. ACS Macro Letters,2012,1:37-40. doi: 10.1021/mz200060z [28] XIANG Z H, CAO D P, WANG W C, et al. Postsynthetic lithium modification of covalent-organic polymers for enhancing hydrogen and carbon dioxide storage[J]. The Journal of Physical Chemistry C,2012,116:5974-5980. doi: 10.1021/jp300137e [29] MA H P, REN H, ZOU X Q, et al. Post metalation of porous aromatic frameworks for highly efficient carbon capture from CO2+N2 and CH4+N2 mixtures[J]. Polymer Chemistry,2014,5:144-152. doi: 10.1039/C3PY00647F [30] WERNER P H. At least 60 years of ferrocene: The discovery and rediscovery of the sandwich complexes[J]. Angewandte Chemie, International Edition,2012,51(25):6025-6058. [31] PETERSON B M, RODNEI B. Electrodegradation of landfill leachate in a flow electmchemical reactor[J]. Chemosphere,2005,58(1):4l-46. [32] SENEL M. Construction of reagentless glucose biosensor based on ferrocene conjugated polypyrrole[J]. Synthetic Metals,2011,161(17-18):1861-1868. doi: 10.1016/j.synthmet.2011.06.025 [33] LAWAL A T, ADELOJU S B. Mediated xanthine oxidase potentiometric biosensors for hypoxanthine based on ferrocene carboxylic acid modified electrode[J]. Food Che-mistry,2012,135(4):2982-2987. doi: 10.1016/j.foodchem.2012.07.052 [34] YASHINA A S. Anthraquinone-ferrocene film electrodes: Utility in pH and oxygen sensing[J]. Electrochemistry Communications,2008,10(12):1831-1834. doi: 10.1016/j.elecom.2008.09.031 [35] 夏碧江, 方嘉炜, 黎姗, 等. 卟啉基多孔有机聚合物的合成、表征及其气体吸附性能研究[J]. 材料导报, 2018, 32(31):144-148.XIA B J, FANG J W, LI S, et al. Synthesis, characterization and gas adsorption properties of porphyrin-based porous organic ploymers[J]. Materials Reports,2018,32(31):144-148(in Chinese). [36] HASMUKH A, PATEL J, SANG H, et al. Unprecedented high-temperature CO2 selectivity in N2-phobic nanoporous covalent organic polymers[J]. Nature Communications,2013,4:1357. doi: 10.1038/ncomms2359 [37] THOMMES M, KANEKO K, NEIMARK A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry,2015,87(9-10):1051-1069. doi: 10.1515/pac-2014-1117 [38] MAKHSEED S, SAMUEL J, BUMAJDAD A, et al. Synthesis and characterization of fluoropolymers with intrinsic microporosity and their hydrogen adsorption studies[J]. Journal of Applied Polymer Science,2008,109(4):2591-2597. doi: 10.1002/app.28372 [39] GAO H, DING L, LI W, et al. Hyper-cross-linked organic microporous polymers based on alternating copolymerization of bismaleimide[J]. ACS Macro Letters,2016,5:377-381. doi: 10.1021/acsmacrolett.6b00015 [40] TO J W, HE J, MEI J, et al. Hierarchical N-doped carbon as CO2 adsorbent with high CO2 selectivity from rationally designed polypyrrole precursor[J]. Journal of the American Chemical Society,2016,138:1001-1009. doi: 10.1021/jacs.5b11955 [41] FU X, ZHANG Y, GU S, et al. Metal microporous aromatic polymers with improved performance for small gas storage[J]. Chemistry—A European Journal, 2015, 21: 13357-13363. [42] WONG M, BUDA C, DUNIETZ B D. Hydrogen physisorption on the organic linker in metal organic frameworks: Ab initio computational study[J]. Journal of Physical Che-mistry B,2006,110(21):10479-10484. doi: 10.1021/jp710703m [43] ZHANG L, ZHANG H, GUO W, et al. Removal of malachite green and crystal violet cationic dyes from aqueous solution using activated sintering process red mud[J]. Applied Clay Science,2014,93-94:85-93. doi: 10.1016/j.clay.2014.03.004 [44] 龚文朋, 陈丹, 杨水金. 一种阴离子型三维金属有机框架材料Cu(BDC-NH2)(4, 4'-Bipy)(0.5)(BDC=对苯二甲酸根, Bipy=联吡啶)的制备及其对甲基紫的吸附性能[J]. 应用化学, 2017(11):108-115.GONG W P, CHEN D, YANG S J. Adsorption of methyl violet by an anionic metal-organic framework Cu(BDC-NH2) (4, 4'-Bipy)(0.5)(BDC= terephthalicacid, Bipy=bipyridine)[J]. Chinese Journal of Applied Chemistry,2017(11):108-115(in Chinese). [45] 李长珍. 介孔材料CMK-3对甲基紫的吸附性能[J]. 化学研究, 2011, 22(6):61-64. doi: 10.3969/j.issn.1008-1011.2011.06.016LI C Z. Adsorption of methyl violet in aqueous solution by CMK-3 mesoporous materia[J]. Chemical Research,2011,22(6):61-64(in Chinese). doi: 10.3969/j.issn.1008-1011.2011.06.016 [46] 林俊雄. 硅藻土基吸附剂的制备、表征及其染料吸附特性研究[D]. 杭州: 浙江大学, 2007.LIN J X. Study on preparation, characterization and dyes adsorption properties of diatomite-based adsorbent[D]. Hangzhou: Zhejiang University, 2007(in Chinese). [47] 刘德坤, 刘航, 杨柳, 等. 镧、铈改性介孔氧化铝对氟离子的吸附[J]. 材料导报, 2019, 33(4):590-594. doi: 10.11896/cldb.201904005LIU D K, LIU H, YANG L, et al. Adsorption of fluoride ions by modified mesoporous alumina with lanthanum and cerium[J]. Materials Guide,2019,33(4):590-594(in Chinese). doi: 10.11896/cldb.201904005 [48] 谭国炽, 李苏哲, 陈逸凡, 等. KMnO4和KOH改性椰壳生物炭的制备及其去除废水中铀的机理研究[J]. 南华大学学报(自然科学版), 2021, 35(5):1-6.TAN G C, LI S Z, CHEN Y F, et al. Preparation of KMnO4 and KOH modified coconut biochar and its adsorption mechanism of U(VI) from wastewater[J]. Journal of University of South China (Science and Technology),2021,35(5):1-6(in Chinese). -





 下载:
下载: