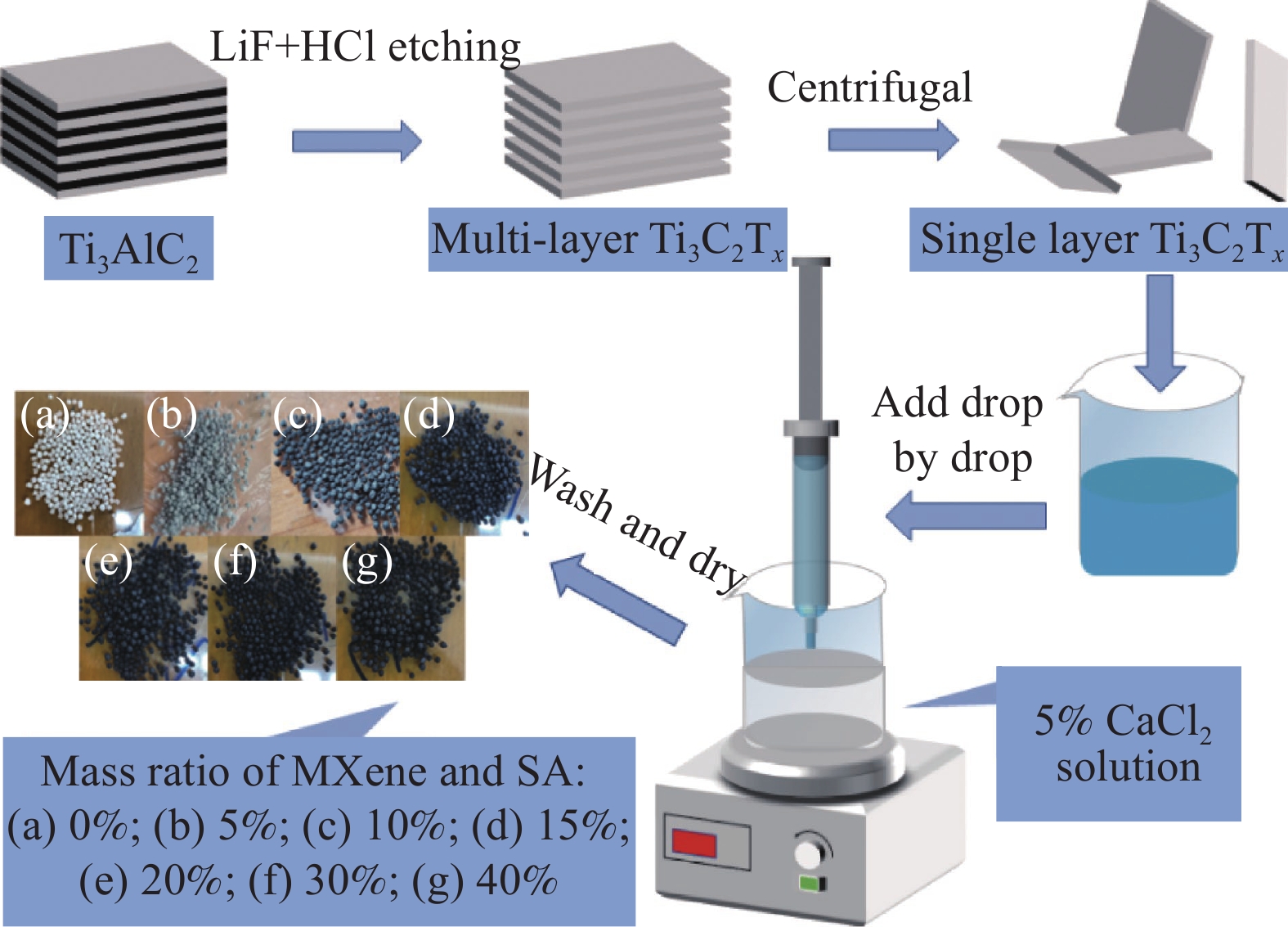
Preparation of MXene/SA gel microspheres and its adsorption performance for U(VI)
-
摘要: 为了提高纳米材料MXene的吸附能力和可回收性,采用离子交联法将海藻酸钠(SA)和MXene混合,使Ti3C2Tx MXene纳米材料固定在SA凝胶基质上,经冷冻干燥后制备了MXene/SA凝胶微球。通过SEM-EDS、FTIR和XPS对凝胶微球结构进行了表征,并考察了不同因素影响下MXene/SA凝胶微球对水溶液中铀(VI)的吸附特性,并探究了其循环再生能力。结果表明,MXene/SA凝胶微球对铀吸附过程遵循拟二级动力学和Langmuir等温吸附模型,说明该吸附主要为单分子层化学吸附,且热力学参数表明其吸附过程是一个自发吸热的过程。在pH为4,温度为298 K时,MXene/SA凝胶微球对铀的最大吸附量为126.82 mg·g−1,其主要吸附机制是离子交换与络合作用。更重要的是该凝胶微球经5次循环后,去除率仍然保持在90%以上,说明吸附剂具有回收再生利用性能,且不会造成水环境的二次污染。因此,MXene/SA凝胶微球吸附剂在修复放射性核素铀废水污染方面表现出巨大潜力。Abstract: In order to improve the adsorption capacity and recyclability of the nanomaterial MXene, sodium alginate (SA) and MXene were mixed by ion cross-linking method to fix the Ti3C2Tx MXene nanomaterial on the SA aerogel matrix. After freeze-drying, MXene/SA gel microspheres was prepared. The structure of the gel microspheres was characterized by SEM-EDS, FTIR and XPS, and the adsorption characteristics of MXene/SA gel microspheres to uranium (VI) in aqueous solution were investigated under the influence of different factors, and its recycling ability was explored. The results show that the adsorption of uranium by MXene/SA gel microspheres follows the pseudo-second-order kinetics and Langmuir isotherm adsorption model, indicating that the adsorption is mainly monolayer chemical adsorption, and the thermodynamic parameters indicate that the adsorption process is a spontaneous endothermic. When the pH is 4 and the temperature is 298 K, the maximum adsorption capacity of MXene/SA gel microspheres for uranium is 126.82 mg·g−1. The main adsorption mechanism is ion exchange and complexation. More importantly, after 5 cycles of the gel microspheres, the removal rate remains above 90%, indicating that the adsorbent has the performance of recycling and reuse and will not cause secondary pollution to the water environment. Therefore, MXene/SA gel microsphere adsorbent has shown great potential in repairing the pollution of radionuclide uranium wastewater.
-
Key words:
- MXene /
- sodium alginate /
- gel microspheres /
- uranium /
- adsorption performance
-
图 2 Ti3C2Tx (a)、SA凝胶微球 (b)、MXene/SA凝胶微球吸附U(VI)前 (c) 和吸附后 (d) 的SEM图像;MXene/SA凝胶微球吸附U(VI)前 (e) 和吸附后 (f) 的EDS图谱
Figure 2. SEM images of Ti3C2Tx (a), SA gel microspheres (b), MXene/SA gel microspheres before (c) and after (d) adsorption; EDS patterns of MXene/SA gel microspheres before (e) and after (f) adsorption
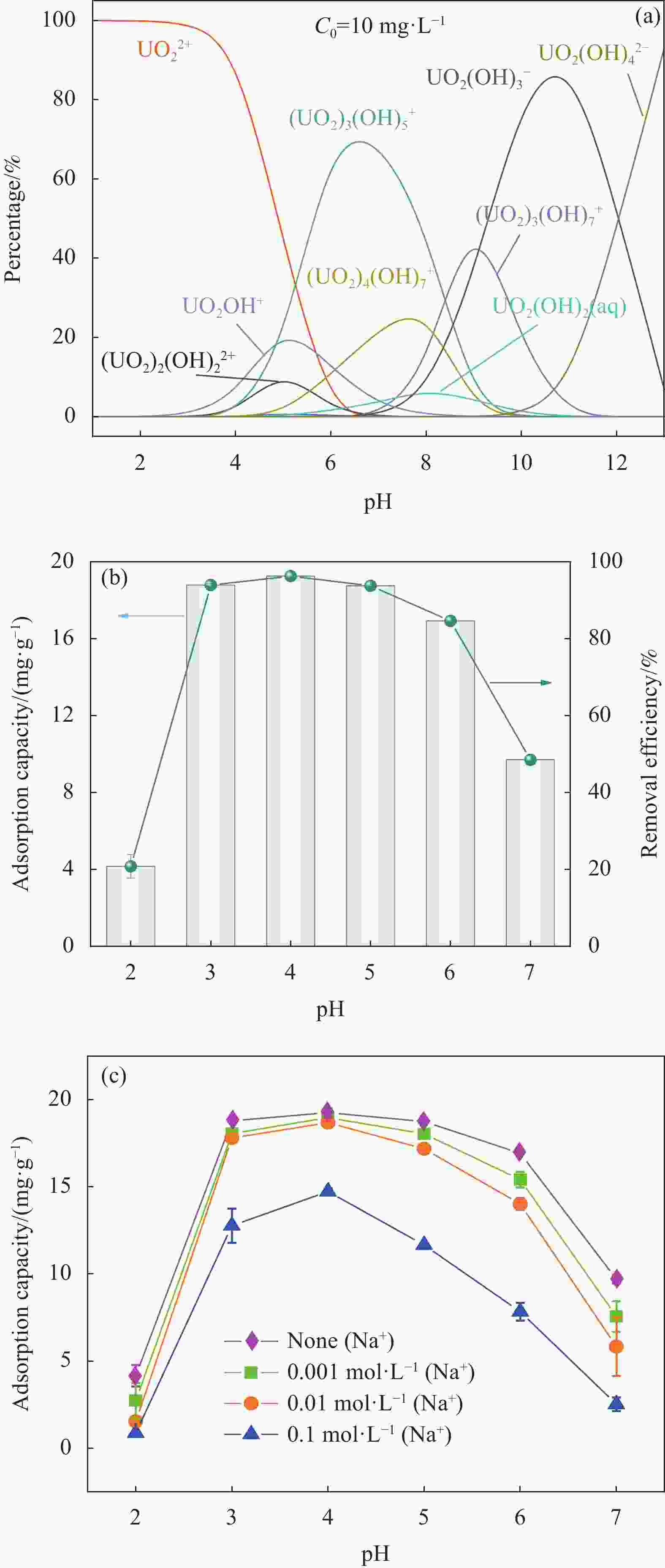
图 6 (a) 不同pH值下U(VI)形态分布曲线图;(b) 不同pH值下MXene/SA对U(VI)吸附性能的影响; (c) 不同硝酸钠离子强度下对MXene/SA吸附U(VI)的影响
Figure 6. (a) U(VI) morphology distribution curves at different pH values; (b) Effect of MXene/SA on the adsorption performance of U(VI) at different pH values; (c) Effect of adsorption U(VI) of MXene/SA adsorbent under different sodium nitrate ionic strength
C0—Initial U(VI) concentration
图 8 (a) 接触时间对MXene/SA凝胶微球吸附不同浓度U(VI)的影响;(b) 拟一级动力学模型拟合曲线; (c) 拟二级动力学模型拟合曲线;(d) 颗粒内扩散模型拟合曲线
Figure 8. (a) Influence of contact time on the adsorption of different concentrations of U(VI) by MXene/SA gel microspheres; (b) Quasi-first-order adsorption kinetic model fitting curves; (c) Quasi-second-order adsorption kinetic model fitting curves; (d) Intra-particle diffusion model fitting curves
qt—Adsorption capacity at time t; qe—Equilibrium adsorption capacity; t—Adsorption time
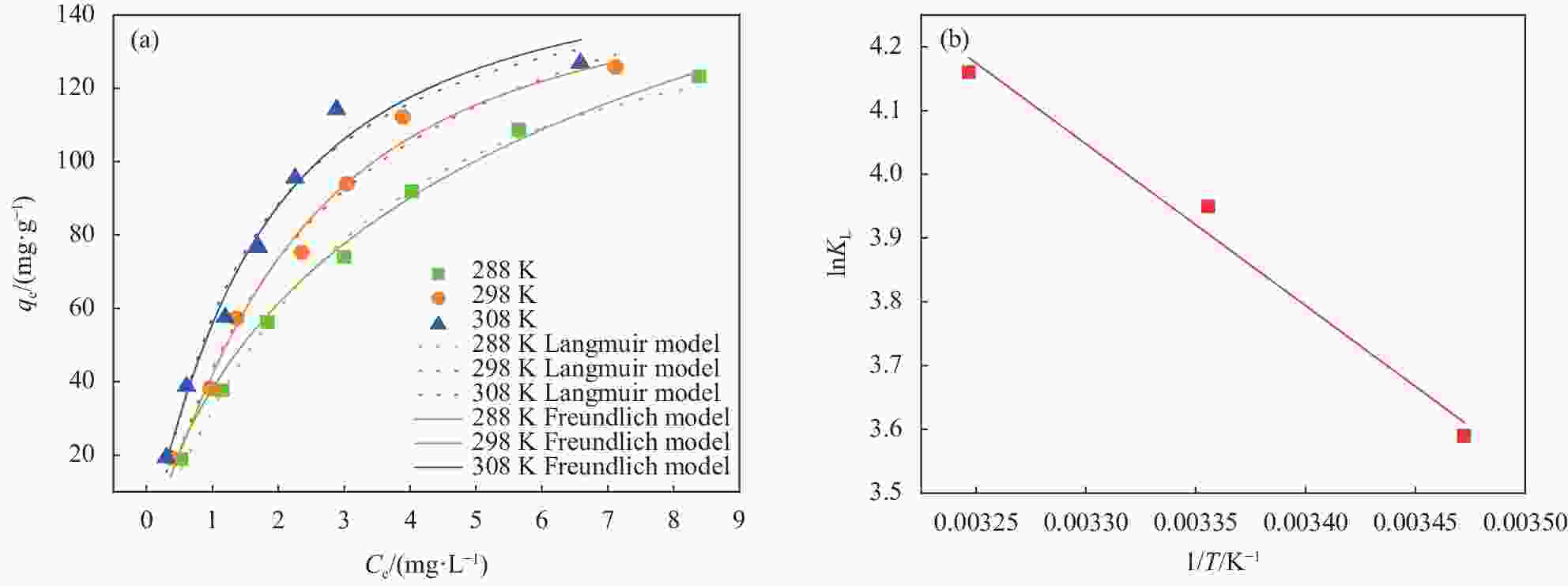
图 9 (a) MXene/SA吸附 U(VI)的非线性等温模型拟合;(b) lnKL与 1/T 的关系图
Figure 9. (a) Fitting of nonlinear isothermal model of MXene/SA adsorption of U(VI); (b) Relationship between lnKL and 1/T
qe—Equilibrium adsorption capacity; Ce—Uranium concentration at adsorption equilibrium; KL—Langmuir coefficient related to the affinity of binding site; T—Temperature
表 1 MXene/SA凝胶微球对U(VI)的吸附动力学参数
Table 1. The adsorption kinetic parameters of U(VI) on MXene/SA gel microspheres
C0/(mg·L−1) 5 10 15 qe,exp/(mg·g−1) 9.7 19.28 28.5 Pseudo-first-order model k1/min−1 0.018 0.016 0.014 qe,cal/(mg·g−1) 9.851 19.426 28.509 R2 0.877 0.853 0.975 Pseudo-second-order model k2/min−1 0.002 0.001 0.001 qe,cal/(mg·g−1) 11.072 21.966 32.783 R2 0.997 0.995 0.996 Intraparticle diffusion model k1p/(mg·(g·min0.5)−1) 0.872 1.745 2.459 C1 −0.774 −2.069 −3.352 R12 0.997 0.942 0.930 k2p/(mg·(g·min0.5)−1) 0.126 0.529 1.010 C2 7.746 11.207 11.764 R22 0.720 0.665 0.963 k3p/(mg·(g·min0.5)−1) 0.028 0.020 0.075 C3 9.203 18.856 26.896 R32 0.652 0.575 0.590 Notes: qe,exp—Calculated amount of adsorption equilibrium; qe,cal—Actual amount of adsorption equilibrium; k1 and k2—First order rate constant and second order rate constant, respectively; kp1, kp2, kp3—Particle diffusion constant; R2—Correlation coefficient. 表 2 Langmuir和Freundlich吸附等温线模型的相关参数
Table 2. Related parameters of Langmuir and Freundlich adsorption isotherm simulation
T/K Langmuir isotherm Freundlich isotherm qmax/(mg·g−1) KL/(L·mg−1) R2 KF/(L·mg−1) n R2 288 150.382 0.273 0.994 38.123 0.147 0.991 298 154.309 0.373 0.984 43.581 0.241 0.974 308 159.031 0.539 0.975 57.381 0.300 0.963 Notes: qmax—Adsorption capacity per unit mass of the adsorbent; KL—Langmuir coefficient related to the affinity of binding site; KF and n—Constants that are related to the adsorption capacity and the adsorption intensity, respectively. 表 3 MXene/SA吸附U(VI)的热力学参数
Table 3. Thermodynamic parameters of MXene/SA adsorption of U(VI)
T/K lnKe ΔG0/(kJ·mol−1) ΔH0/(kJ·mol−1) ΔS0/(J·(mol·K)−1) 288 3.59 −8.60 21.07 103.18 298 3.95 −9.79 308 4.16 −10.65 Notes: T—Thermodynamic temperature; Ke—Equilibrium constant at different temperatures; ΔH0—Standard enthalpy change; ΔG0—Standard free energy change; ΔS0—Standard entropy change. -
[1] XIE Y, CHEN C, REN X, et al. Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation[J]. Progress in Materials Science,2019,103:180-234. doi: 10.1016/j.pmatsci.2019.01.005 [2] WANG X, CHEN L, WANG L, et al. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides[J]. Science China Chemistry,2019,62(8):933-967. doi: 10.1007/s11426-019-9492-4 [3] ZAHERI P, DAVARKHAH R. Selective separation of uranium from sulfuric acid media using a polymer inclusion membrane containing alamine336[J]. Chemical Papers,2020,74(8):2573-2581. doi: 10.1007/s11696-019-01029-9 [4] ORREGO P, HERNANDEZ J, REYES A. Uranium and molybdenum recovery from copper leaching solutions using ion exchange[J]. Hydrometallurgy,2019,184:116-122. doi: 10.1016/j.hydromet.2018.12.021 [5] OROZCO I, ROMERO M, LARA R, et al. Precipitation of uranium from alkaline liqueurs[J]. Materia (Rio de Janeiro),2018,23(2):e12008. doi: 10.1590/S1517-707620180002.0345 [6] BERGER C, MARIE C, GUILLAUMONT D, et al. Extraction of uranium(VI) and plutonium(IV) with tetra-alkylcarbamides[J]. Solvent Extraction and Ion Exchange,2019,37(2):111-125. doi: 10.1080/07366299.2019.1630095 [7] YU S, MA J, SHI Y, et al. Uranium(VI) adsorption on montmorillonite colloid[J]. Journal of Radioanalytical and Nu-clear Chemistry,2020,324(2):541-549. doi: 10.1007/s10967-020-07083-y [8] CHEN S, HU J, HAN S, et al. A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade[J]. Separation and Purification Technology,2020,251:117340. doi: 10.1016/j.seppur.2020.117340 [9] NAGUIB M, KURTOGLU M, PRESSER V, et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2[J]. Advanced Materials,2011,23(37):4248-4253. doi: 10.1002/adma.201102306 [10] ALHABEB M, MALESKI K, MATHIS T S, et al. Selective etching of silicon from Ti3SiC2 (MAX) to obtain 2D titanium carbide (MXene)[J]. Angewandte Chemie International Edition,2018,57(19):5444-5448. doi: 10.1002/anie.201802232 [11] HAN M, YIN X, LI X, et al. Laminated and two-dimensional carbon-supported microwave absorbers derived from MXenes[J]. ACS Applied Materials & Interfaces,2017,9(23):20038-20045. doi: 10.1021/acsami.7b04602 [12] SHAHZAD A, RASOOL K, MIRAN W, et al. Two-dimensional Ti3C2Tx MXene nanosheets for efficient copper removal from water[J]. ACS Sustainable Chemistry & Engineering,2017,5(12):11481-11488. doi: 10.1021/acssuschemeng.7b02695 [13] ANASORI B, LUKATSKAYA M R, GOGOTSI Y. 2D metal carbides and nitrides (MXenes) for energy storage[J]. Nature Reviews Materials,2017,2(2):16098. doi: 10.1038/natrevmats.2016.98 [14] ZHANG Y J, ZHOU Z J, LAN J H, et al. Theoretical insights into the uranyl adsorption behavior on vanadium carbide MXene[J]. Applied Surface Science,2017,426:572-578. doi: 10.1016/j.apsusc.2017.07.227 [15] HANTANASIRISAKUL K, GOGOTSI Y. Electronic and optical properties of 2D transition metal carbides and nitrides (MXenes)[J]. Advanced Materials,2018,30(52):1804779. doi: 10.1002/adma.201804779 [16] WANG L, SONG H, YUAN L, et al. Efficient U(VI) reduction and sequestration by Ti2CTx MXene[J]. Environmental Science & Technology,2018,52(18):10748-10756. doi: 10.1021/acs.est.8b03711 [17] POGREBNJAK A, SUKHODUB L, SUKHODUB L, et al. Composite material with nanoscale architecture based on bioapatite, sodium alginate and ZnO microparticles[J]. Ceramics International,2019,45(6):7504-7514. doi: 10.1016/j.ceramint.2019.01.043 [18] JIAO C, XIONG J, TAO J, et al. Sodium alginate/graphene oxide aerogel with enhanced strength-toughness and its heavy metal adsorption study[J]. International Journal of Biological Macromolecules,2016,83:133-141. doi: 10.1016/j.ijbiomac.2015.11.061 [19] 朱韵伊, 彭伟, 林泽慧, 等. MXene基水凝胶复合材料的研究进展[J]. 复合材料学报, 2021, 38(7):2010-2024. doi: 10.13801/j.cnki.fhclxb.20210302.004ZHU Yunyi, PENG Wei, LIN Zehui, et al. Research progress of MXene-based hydrogel composites[J]. Acta Materiae Compositae Sinica,2021,38(7):2010-2024(in Chinese). doi: 10.13801/j.cnki.fhclxb.20210302.004 [20] ZHANG T, ZHANG W, XI H, et al. Polydopamine functionalized cellulose-MXene composite aerogel with superior adsorption of methylene blue[J]. Cellulose,2021,28(7):4281-4293. doi: 10.1007/s10570-021-03737-6 [21] SHAHZAD A, NAWAZ M, MOZTAHIDA M, et al. Ti3C2Tx MXene core-shell spheres for ultrahigh removal of mercuric ions[J]. Chemical Engineering Journal,2019,368:400-408. doi: 10.1016/j.cej.2019.02.160 [22] FENG Y, WANG H, XU J, et al. Fabrication of MXene/PEI functionalized sodium alginate aerogel and its excellent adsorption behavior for Cr(VI) and Congo Red from aqueous solution[J]. Journal of Hazardous Materials,2021,416:125777. doi: 10.1016/j.jhazmat.2021.125777 [23] ZHANG Z H, XU J Y, YANG X L. MXene/sodium alginate gel beads for adsorption of methylene blue[J]. Materials Chemistry and Physics,2021,260:124123. doi: 10.1016/j.matchemphys.2020.124123 [24] 郭成, 郝军杰, 李明阳, 等. 海藻酸钠/聚乙烯亚胺凝胶球的合成及对Cr(VI)的吸附性能和机制[J]. 复合材料学报, 2021, 38(7):2140-2151. doi: 10.13801/j.cnki.fhclxb.20201015.003GUO Cheng, HAO Junjie, LI Mingyang, et al. Adsorption of Cr(VI) on porous sodium alginate/polyethyleneimine hydrogel beads and its mechanistic study[J]. Acta Materiae Compositae Sinica,2021,38(7):2140-2151(in Chinese). doi: 10.13801/j.cnki.fhclxb.20201015.003 [25] YI X, SUN F, HAN Z, et al. Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu(II) and U(VI) removal[J]. Ecotoxicology and Envi-ronmental Safety,2018,158:309-318. doi: 10.1016/j.ecoenv.2018.04.039 [26] LIU H, ZHOU Y, YANG Y, et al. Synthesis of polyethylenimine/graphene oxide for the adsorption of U(VI) from aqueous solution[J]. Applied Surface Science,2019,471:88-95. doi: 10.1016/j.apsusc.2018.11.231 [27] ZHANG W, WANG H, HU X, et al. Multicavity triethylenetetramine-chitosan/alginate composite beads for enhanced Cr(VI) removal[J]. Journal of Cleaner Production,2019,231:733-745. doi: 10.1016/j.jclepro.2019.05.219 [28] SHAHZAD A, MOZTAHIDA M, TAHIR K, et al. Highly effective prussian blue-coated MXene aerogel spheres for selective removal of cesium ions[J]. Journal of Nuclear Materials,2020,539:152277. doi: 10.1016/j.jnucmat.2020.152277 [29] JUN B M, JANG M, PARK C M, et al. Selective adsorption of Cs+ by MXene (Ti3C2Tx) from model low-level radioactive wastewater[J]. Nuclear Engineering and Technology,2020,52(6):1201-1207. doi: 10.1016/j.net.2019.11.020 [30] ZHANG P, WANG L, DU K, et al. Effective removal of U(VI) and Eu(III) by carboxyl functionalized MXene nanosheets[J]. Journal of Hazardous Materials,2020,396:122731. doi: 10.1016/j.jhazmat.2020.122731 [31] 郑骁, 王学松, 陈光, 等. 离子强度和pH对针铁矿吸附水溶液中Cd(Ⅱ)的影响[J]. 环境工程, 2019, 37(7):119-123, 208. doi: 10.13205/j.hjgc.201907022ZHENG Xiao, WANG Xuesong, CHEN Guang, et al. Effect of ionic strength and pH on adsorption of Cd(Ⅱ) on Goethite from aqueous solution[J]. Environmental Engineering,2019,37(7):119-123, 208(in Chinese). doi: 10.13205/j.hjgc.201907022 [32] 伍随意, 李仕友, 胡俊毅, 等. 聚乙烯亚胺改性磁性酵母复合材料去除铀(VI)的性能[J]. 复合材料学报, 2021, 38(9):3065-3075.WU Suiyi, LI Shiyou, HU Junyi, et al. Adsorption properties of polyethyleneimine modified magnetic yeast composites for uranium(VI)[J]. Acta Materiae Compositae Sinica,2021,38(9):3065-3075(in Chinese). [33] ZHANG W, SONG J, HE Q, et al. Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(II) removal[J]. Journal of Hazardous Materials,2020,384:121445. doi: 10.1016/j.jhazmat.2019.121445 -





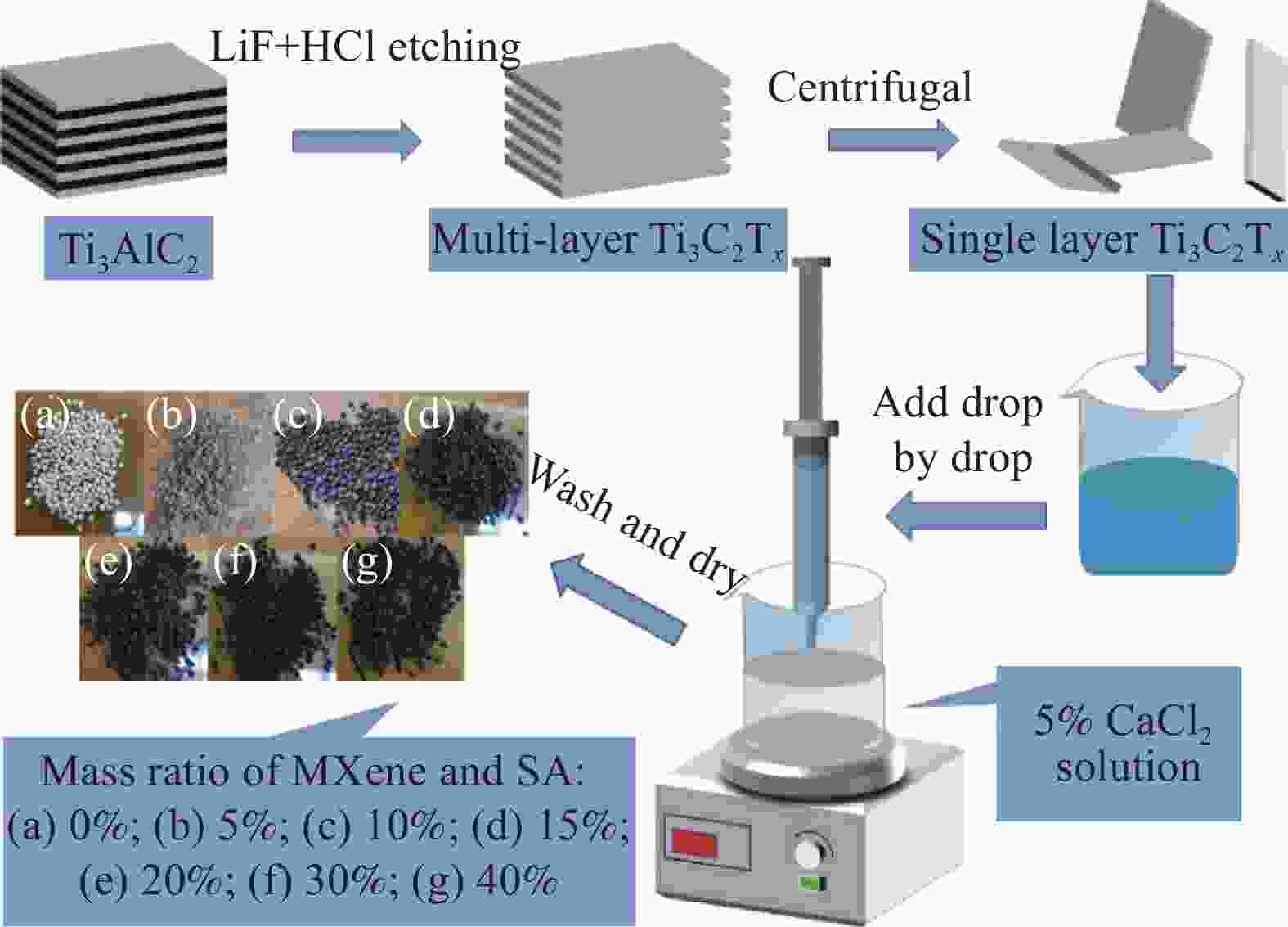
 下载:
下载: