Surface network modification of carbon nanofibers and its application in zinc ion batteries
-
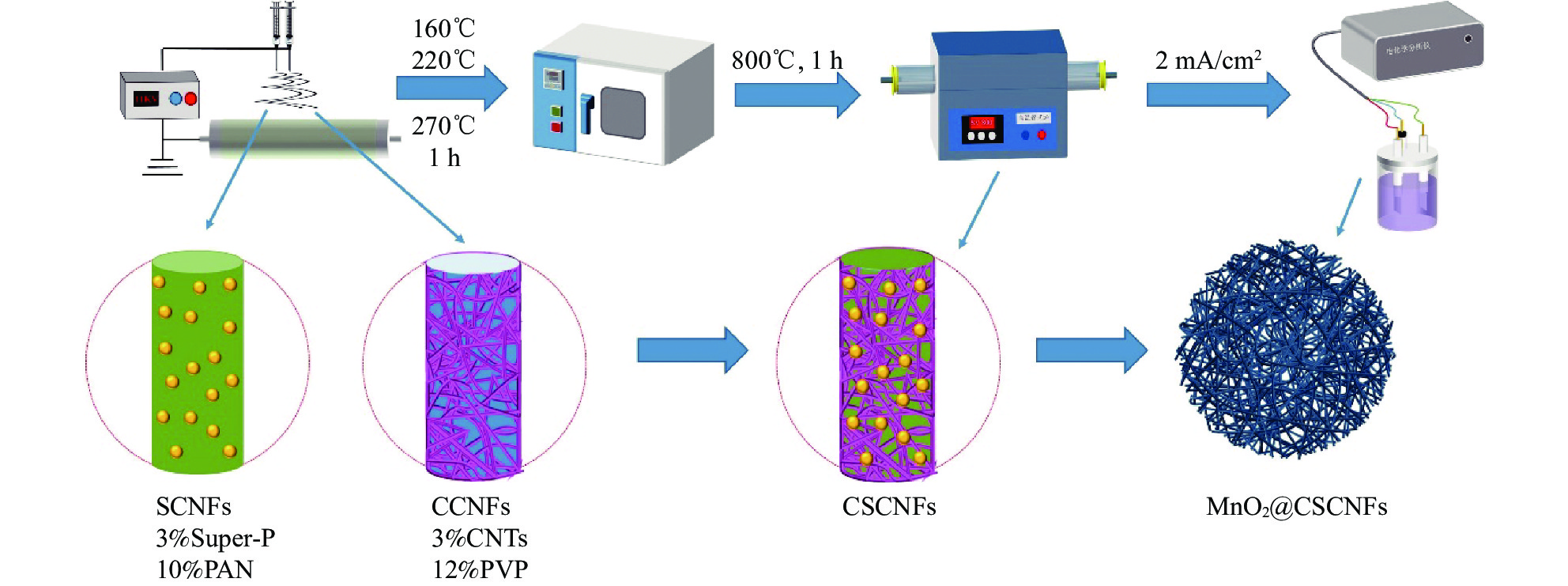
摘要: 可充电水系锌锰电池以高安全、低成本和对环境友好的特性在大规模储能领域有广泛的应用前景,但由于锰氧化合物自身导电差且在电池充放电过程中发生歧化反应在水中溶解,导致电池容量低、循环稳定性差。本文采用双针头对纺静电纺丝技术,结合预氧化、高温退火工艺,通过掺杂碳纳米管(CNTs)和导电炭黑(Super-P)对碳纳米纤维表面进行修饰,制备出具有凸起结构和导电网络的碳纳米纤维(CSCNFs)复合材料,再结合电化学沉积工艺,在纤维表面负载α-MnO2活性物质制备得到MnO2@CSCNFs阴极。其中,CNTs和Super-P协同构建了具有节点结构的导电网络通道,实现高效电子-离子协同传输。以MnO2@CSCNFs为阴极的电化学性能得到明显改善,初始容量达到784.8 mA·h·g−1,100圈循环后仍保持500 mA·h·g−1的放电比容量,2 A·g−1的大电流密度下仍保持290.8 mA·h·g−1的放电比容量,且当电流密度恢复到0.1 A·g−1时容量回复率高达96.33%。
-
关键词:
- 可充电水系锌离子电池 /
- 静电纺丝技术 /
- α-MnO2 /
- 阴极材料 /
- 表面修饰
Abstract: Rechargeable water zinc-manganese battery has a wide application prospect in large-scale energy storage due to its high safety, low cost and environmental friendliness. However, due to poor conductivity of manganese oxide and dissolving in water due to disproportionation reaction during battery charging and discharging, the battery has low capacity and poor cycle stability. In this paper, the carbon nanofiber (CSCNFs) composite material with raised structure and conductive network was prepared by double-needle pair spinning electrostatic spinning technology, combined with pre-oxidation and high temperature annealing process, and the surface of carbon nanofiber was modified by doping carbon nanotube (CNTs) and conductive carbon black (Super-P). MnO2@CSCNFs cathode was prepared by loading α-MnO2 active substance on the fiber surface. CNTs and Super-P doping were modified on the surface of carbon nanofibers. Among them, CNTs and Super-P cooperated to construct conductive network channels with node structure to realize efficient electron-ion cooperative transport. With the cathode of MnO2@CSCNFs zinc ion battery kinetics and electrochemical performance is significantly improved, the initial capacity reaches 784.8 mA·h·g−1, and after 100 cycle remain discharge specific capacity of 500 mA·h·g−1. The discharge specific capacity of 290.8 mA·h·g−1 is maintained at a high current density of 2 A·g−1, and the capacity recovery rate is up to 96.33% when the current density is restored to 0.1 A·g−1. -
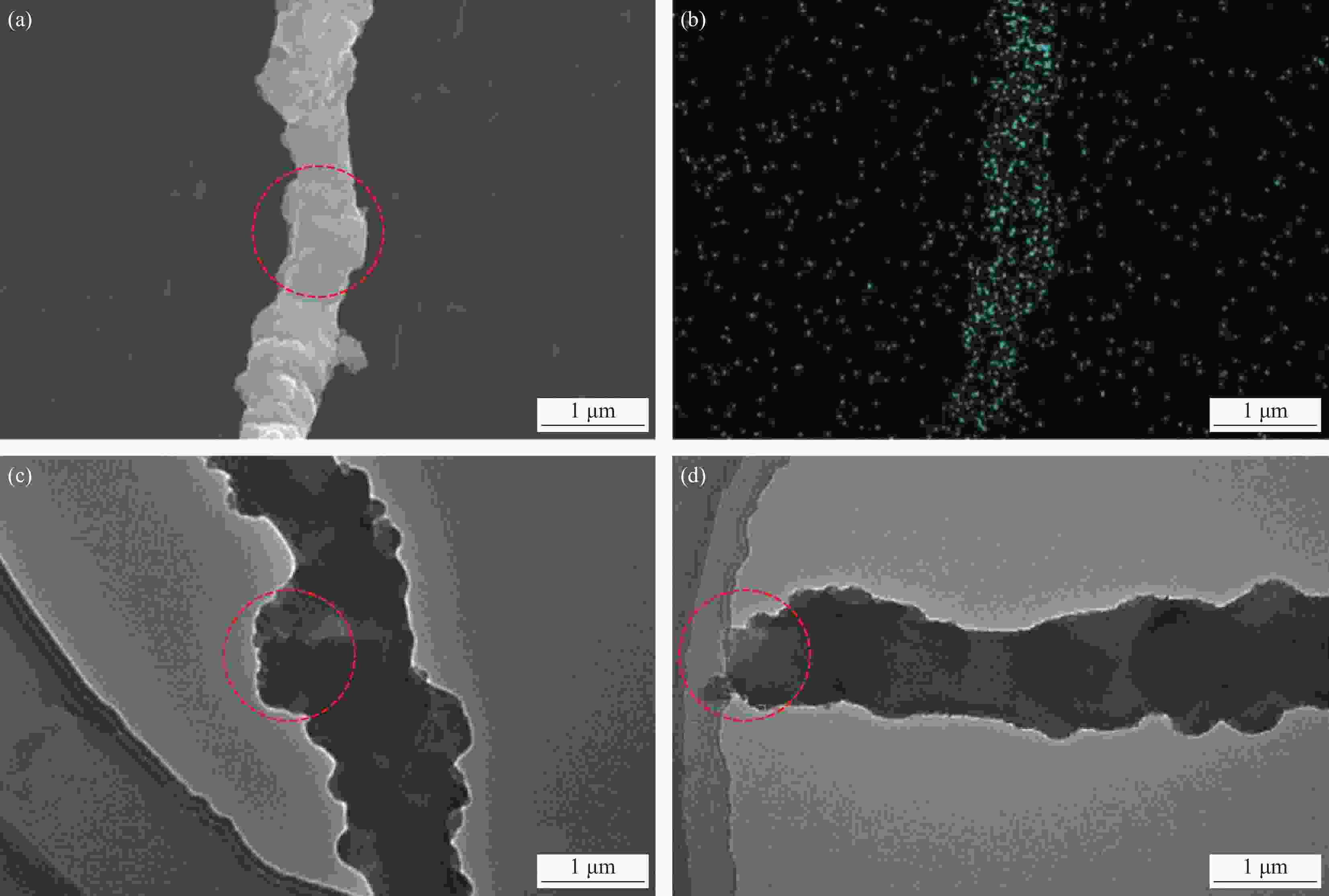
图 2 CCNFs、SCNFs、CSCNFs碳化前((a)~(c))及高温退火后((a1)~(c1))的SEM图像;((a2)~(c2)) MnO2@CCNFs、MnO2@SCNFs、MnO2@CSCNFs的SEM图像
Figure 2. SEM images of CCNFs, SCNFs and CSCNFs before carbonization ((a)-(c)) and after high temperature annealing ((a1)-(c1)); ((a2)-(c2)) SEM images of MnO2@CCNFs, MnO2@SCNFs and MnO2@CSCNFs
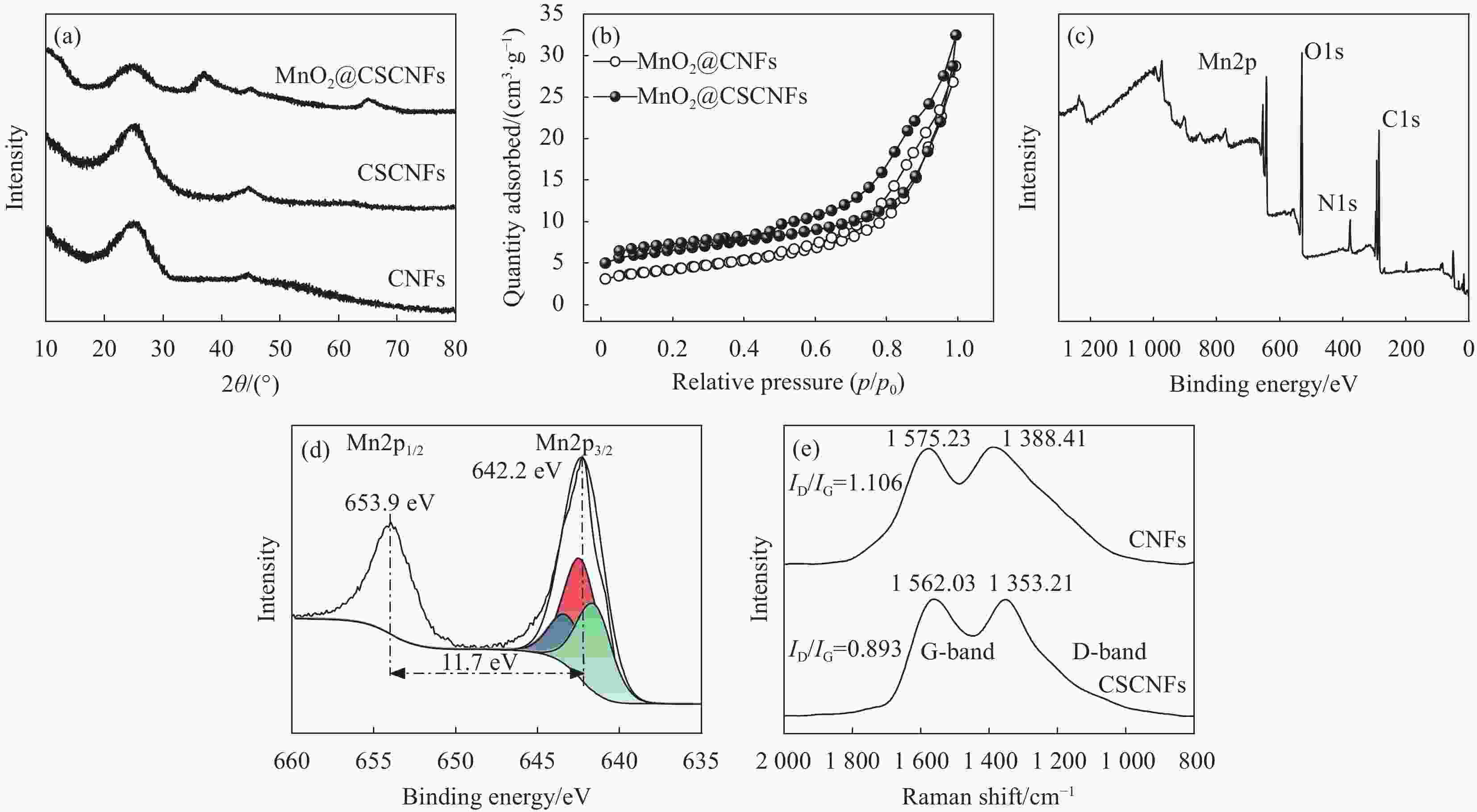
图 4 (a) CNFs、CSCNFs和MnO2@CSCNF的XRD图谱;(b) MnO2@CNFs和MnO2@CSCNFs氮气吸附-脱附等温曲线;(c) MnO2@CSCNFs复合材料XPS图谱;(d) MnO2@CSCNFs复合材料Mn2p图谱;(e) CNFs和CSCNFs的Raman图谱
ID/IG—Intensity ratio between D and G bands
Figure 4. (a) XRD patterns of CNFs, CSCNFs and MnO2@CSCNFs; (b) Nitrogen adsorption-desorption isotherm curves of MnO2@CNFs and MnO2@CSCNFs; (c) XPS survey spectrum of MnO2@CSCNFs; (d) Mn2p XPS spectrum of MnO2@CSCNFs; (e) Raman spectra of CNFs and CSCNFs
图 5 (a) MnO2@CSCNFs复合阴极的CV曲线;(b) 0.1 A·g−1电流密度下MnO2@CCNFs、MnO2@SCNFs和MnO2@CSCNFs的充放电曲线;(c) MnO2@CCNFs、MnO2@SCNFs、MnO2@CSCNFs在不同电流密度下的倍率性能曲线;(d) 0.1 A·g−1电流密度下MnO2@CCNFs、MnO2@SCNFs、MnO2@CSCNFs自支撑阴极前100次循环曲线;(e) 1 A·g−1电流密度下1000圈循环曲线;(f) 2 A·g−1电流密度下MnO2@CSCNFs的2000圈循环曲线;(g) 电池容量性能比较
Figure 5. (a) CV curves of MnO2@CSCNFs; (b) Charge-discharge curves of MnO2@CCNFs, MnO2@SCNFs and MnO2@CSCNFs at 0.1 A·g−1 current density; (c) Rate performance curves of MnO2@CCNFs, MnO2@SCNFs and MnO2@CSCNFs at different current densities; (d) Cycle performance of MnO2@CCNFs, MnO2@SCNFs and MnO2@CSCNFs at 0.1 A·g−1 current density; (e) 1000 cycle curve at 1 A·g−1 current density; (f) 2000 cycle curve at 2 A·g−1 current density of MnO2@CSCNFs; (g) Battery capacity performance comparison diagram
图 7 (a) MnO2@CSCNFs电极在不同扫速下的CV曲线;(b) 不同峰位lgi-lgv的拟合曲线;(c) 在不同扫速下MnO2@CSCNFs电极电荷存储过程中电容控制的容量百分比;(d) 峰值电流(ip)和扫描速率(v1/2)之间的线性关系;(e) MnO2@CSCNFs电极的恒流间歇滴定(GITT)曲线;(f) MnO2@CSCNFs电极的离子扩散系数
v—Scan rate; D—Diffusion coefficient of ions; k—Specific value of peak currents (ip) and scan rates (v1/2)
Figure 7. (a) CV curves of MnO2@CSCNFs cathode at different scan rates; (b) lgi-lgv plots at specific peak currents; (c) Percentages of the capacitive response in the charge storage process of the MnO2@CSCNFs cathode at different scan rates; (d) Linear relationship between peak currents (ip) and scan rates (v1/2); (e) Constant current batch titration (GITT) curves of MnO2@CSCNFs cathode; (f) Corresponding ion diffusion coefficients of MnO2@CSCNFs cathode
-
[1] ZHOU H J, SONG C L, SI L P, et al. The development of catalyst materials for the advanced lithium-sulfur battery[J]. Catalysts,2020,10(6):682-698. doi: 10.3390/catal10060682 [2] 夏傲, 曾啸雄, 宜珏, 等. Ag/MnO2复合电极材料的制备及其电化学性能[J]. 复合材料学报, 2022, 39(5):2269-2279.XIA Ao, ZENG Xiaoxiong, YI Jue, et al. Preparation and electrochemical properties of Ag/MnO2 composite electrode materials[J]. Acta Materiae Compositae Sinica,2022,39(5):2269-2279(in Chinese). [3] BORCHERS N, CLARK S, HORSTMANN B, et al. Innovative zinc-based batteries[J]. Journal of Power Sources,2021,484:229309. doi: 10.1016/j.jpowsour.2020.229309 [4] HARUDIN N, OSMAN Z, MAJID S R, et al. Improved electrochemical properties of MgMn2O4 cathode materials by Sr doping for Mg ion cells[J]. Ionics,2020,26(8):3947-3958. doi: 10.1007/s11581-020-03531-7 [5] LUO M H, YU H X, HU F Y, et al. Metal selenides for high performance sodium ion batteries[J]. Chemical Engineering Journal,2020,380:122557. doi: 10.1016/j.cej.2019.122557 [6] SMITH B D, WILLS R G A, CRUDEN A J. Aqueous Al-ion cells and supercapacitors–A comparison[J]. Energy Reports,2020,6:166-173. [7] 黄兰香, 罗旭峰. 用于可充电水性锌离子电池的先进Ti3C2@ε-MnO2电极[J]. 复合材料学报, 2022, 39(10):4631-4641.HUANG Lanxiang, LUO Xufeng. Advanced Ti3C2@ε-MnO2 cathode as rechargeable aqueous zinc-ion batteries[J]. Acta Materiae Compositae Sinica,2022,39(10):4631-4641(in Chinese). [8] LIU X, EUCHNER H, ZARRABEITIA M, et al. Operando pH measurements decipher H+/Zn2+ intercalation chemistry in high-performance aqueous Zn/δ-V2O5 batteries[J]. ACS Energy Letters,2020,5(9):2979-2986. doi: 10.1021/acsenergylett.0c01767 [9] ZHANG W H, ZHAI X L, ZHANG Y S, et al. Application of manganese-based materials in aqueous rechargeable zinc-ion batteries[J]. Frontiers in Energy Research,2020,8:00195. doi: 10.3389/fenrg.2020.00195 [10] FAN X Y, YANG H, NI K F, et al. Electrochemical controllable synthesis of MnO2 as cathode of rechargeable zinc-ion battery[J]. Functional Materials Letters,2020,13(3):2050011. doi: 10.1142/S1793604720500113 [11] LEE S Y, WU L J, POYRAZ A S, et al. Lithiation mechanism of tunnel-structured MnO2 electrode investigated by in situ transmission electron microscopy[J]. Advanced Materials,2017,29(43):1703186. doi: 10.1002/adma.201703186 [12] CAI Y, CHUA R, HUANG S Z, et al. Amorphous manganese dioxide with the enhanced pseudocapacitive performance for aqueous rechargeable zinc-ion battery[J]. Chemical Engineering Journal,2020,396:125221. doi: 10.1016/j.cej.2020.125221 [13] HUANG L X, LUO X F, CHEN C, et al. A high specific capacity aqueous zinc-manganese battery with a ε-MnO2 cathode[J]. Ionics,2021,27(9):3933-3941. doi: 10.1007/s11581-021-04160-4 [14] LIU W B, ZHANG X Y, HUANG Y F, et al. β-MnO2 with proton conversion mechanism in rechargeable zinc ion battery[J]. Journal of Energy Chemistry,2021,56:365-373. doi: 10.1016/j.jechem.2020.07.027 [15] ZHAO L, DONG L B, LIU W B, et al. Binary and ternary manganese dioxide composites cathode for aqueous zinc-ion battery[J]. ChemistrySelect,2018,3(44):12661-12665. doi: 10.1002/slct.201802954 [16] TANG X N, ZHU S K, NING J, et al. Charge storage mechanisms of manganese dioxide-based supercapacitors: A review[J]. New Carbon Materials,2021,36(4):702-708. doi: 10.1016/S1872-5805(21)60082-3 [17] WEI X B, YUAN H C, WANG H J, et al. The metal-organic framework mediated synthesis of bell string-like hollow ZnS-C nanofibers to enhance sodium storage performance[J]. Materials Chemistry Frontiers,2021,5(12):4712-4724. doi: 10.1039/D1QM00423A [18] MASSA-ANGKUL N, KNIJNENBURG J T N, KASEMSIRI P, et al. Electrophoretic deposition of carbon nanotubes onto zinc substrates for electrode applications[J]. Sains Malaysiana,2020,49(11):2811-2820. doi: 10.17576/jsm-2020-4911-20 [19] BORUAH B D, MATHIESON A, PARK S K, et al. Vanadium dioxide cathodes for high-rate photo-rechargeable zinc-ion batteries[J]. Advanced Energy Materials,2021,11(13):2100115. doi: 10.1002/aenm.202100115 [20] CANG R B, YE K, ZHU K, et al. Organic 3D interconnected graphene aerogel as cathode materials for high-performance aqueous zinc ion battery[J]. Journal of Energy Chemistry,2020,45:52-58. doi: 10.1016/j.jechem.2019.09.026 [21] ZHOU J H, XIE M, WU F, et al. Ultrathin surface coating of nitrogen-doped graphene enables stable zinc anodes for aqueous zinc-ion batteries[J]. Advanced Materials,2021,33(33):2101649. doi: 10.1002/adma.202101649 [22] YU H, CHEN L, LI W X, et al. Root-whisker structured 3D CNTs-CNFs network based on coaxial electrospinning: A free-standing anode in lithium-ion batteries[J]. Journal of Alloys and Compounds,2021,863:158481. doi: 10.1016/j.jallcom.2020.158481 [23] LI Z X, LIU L, LI L D, et al. In situ synthesis of ZnFe2O4 rough nanospheres on carbon nanofibers as an efficient titanium mesh substrate counter electrode for triiodide reduction in dye-sensitized solar cells[J]. Applied Surface Science,2021,541:148429. doi: 10.1016/j.apsusc.2020.148429 [24] PASCARIU P, HOMOCIANU M. ZnO-based ceramic nanofibers: Preparation, properties and applications[J]. Ceramics International,2019,45(9):11158-11173. doi: 10.1016/j.ceramint.2019.03.113 [25] KONG L Q, LIU H, CAO W Y, et al. PAN fiber diameter effect on the structure of PAN-based carbon fibers[J]. Fibers and Polymers,2014,15(12):2480-2488. doi: 10.1007/s12221-014-2480-1 [26] WU F, GAO X, XU X, et al. Boosted Zn storage performance of MnO2 nanosheet-assembled hollow polyhedron grown on carbon cloth via a facile wet-chemical synthesis[J]. ChemSusChem,2019,13(6):1537-1545. [27] CHEN H, DAI C, XIAO F, et al. Reunderstanding the reaction mechanism of aqueous Zn-Mn batteries with sulfate electrolytes: Role of the zinc sulfate hydroxide[J]. Advanced Materials,2022,34(15):e2109092. doi: 10.1002/adma.202109092 [28] ZHANG Y A, LIU Y P, LIU Z H, et al. MnO2 cathode materials with the improved stability via nitrogen doping for aqueous zinc-ion batteries[J]. Journal of Energy Chemistry,2022,64:23-32. doi: 10.1016/j.jechem.2021.04.046 [29] LI S, LIU Y, ZHAO X, et al. Sandwich-like heterostructures of MoS2 graphene with enlarged interlayer spacing and enhanced hydrophilicity as high-performance cathodes for aqueous zinc-ion batteries[J]. Advanced Materials,2021,33(12):e2007480. doi: 10.1002/adma.202007480 -






 下载:
下载: