Preparation and magnetic properties of sulfonated polystyrene @Fe3O4 magnetic composite particles by self-assembly method
-
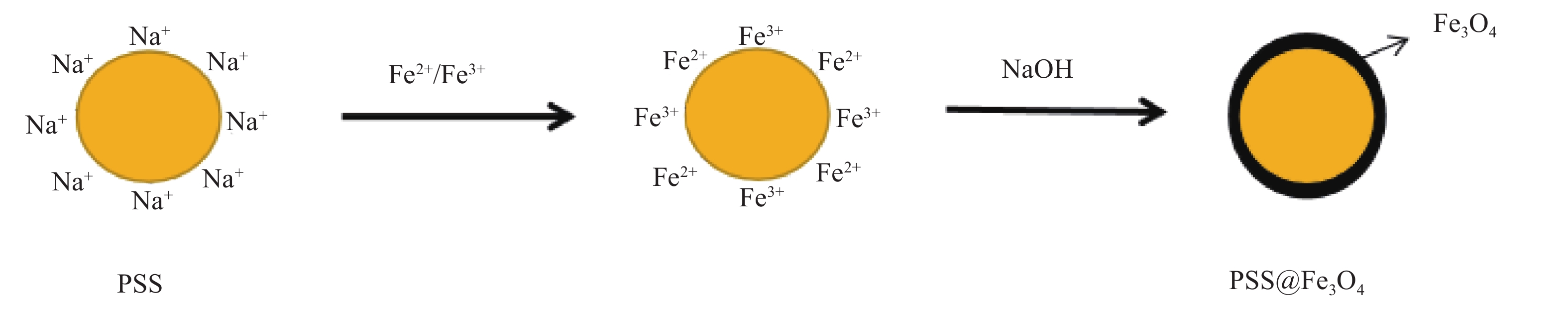
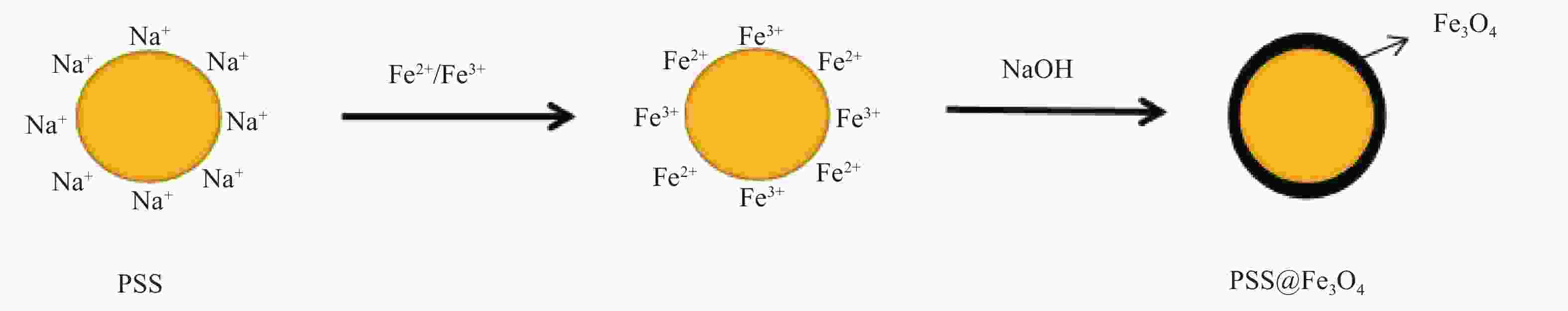
摘要: 实现结构可控、均匀包覆是制备核-壳复合材料的关键。采用离子交换法完成了磺化聚苯乙烯(PSS)表面Na+与溶液中Fe2+和Fe3+的交换,于碱性条件下制备了PSS表面负载Fe3O4(PSS@Fe3O4)的磁性复合颗粒。通过称重法计算了Fe3O4最大包覆率;通过振动样品磁强计(VSM)测试了不同负载含量下PSS@Fe3O4复合颗粒的磁性能;通过XRD、衰减全反射-FTIR (ATR-FTIR)、SEM-EDS分析了PSS@Fe3O4磁性复合颗粒的化学组成和微观结构。结果表明,随着Fe2+/Fe3+浓度增加,PSS@Fe3O4磁性颗粒的饱和磁化强度也随之增大,最大饱和磁化强度为7.51 emu/g,并具有明显的磁响应性;Fe3O4均匀包覆在PSS表面,最大包覆率为8.3 wt%。PSS@Fe3O4磁性复合颗粒有望用于磁流变、医学及水处理领域。Abstract: Achieving controllable structure and uniform coating is the key for the preparation of core-shell magnetic composite materials. The exchange of Na+ with Fe2+ and Fe3+ on the surface of sulfonic polystyrene (PSS) was completed by ion exchange method. The PSS surface loaded Fe3O4(PSS@Fe3O4) magnetic composite particles was obtained with alkaline existed. The maximum encapsulation rate of Fe3O4 was calculated by weighing method. The magnetic properties were analyzed by vibrating sample magnetometer (VSM). The chemical composition and microstructure of PSS@ Fe3O4 magnetic composite particles were analyzed by XRD, ATR-FTIR and SEM-EDS. The results show that the saturation magnetization of PSS@Fe3O4 magnetic particles increases with the increase of Fe2+/Fe3+ concentration. The maximum saturation magnetization of PSS@Fe3O4 magnetic particles is 7.51 emu/g which owned the obvious magnetic response. Fe3O4 is evenly coated on the surface of PSS, and the maximum coating rate is 8.3 wt%. The PSS@Fe3O4 composite particles are expected to be applied in magnetorheological, medical and waste water treatment fields.
-
Key words:
- self-assemble method /
- sulfonic polystyrene /
- Fe3O4 /
- magnetic properties /
- composite particle
-
表 1 制备PSS@Fe3O4磁性复合颗粒的反应物比例
Table 1. Reaction recipes of the preparation of PSS@Fe3O4 magnetic composite particles
Sample FeCl2·4H2O/g FeCl3·6H2O/g PSS/g NaOH/(mol·L−1) Cladding mass fraction/wt% 1 0.30 0.2 5.82 1.25 1.3 2 0.60 0.4 5.82 1.25 2.5 3 0.90 0.6 5.82 1.25 4.3 4 1.20 0.8 5.82 1.25 5.4 5 1.50 1.0 5.82 1.25 6.8 6 2.25 1.5 5.82 1.25 7.7 7 3.00 2.0 5.82 1.25 8.3 8 4.50 3.0 5.82 1.25 8.3 9 7.50 5.0 5.82 1.25 8.3 -
[1] 杨琥, 袁博, 卢耀柏, 等. 壳聚糖磁性微球的制备及在水处理中的应用[J]. 中国科学, 2008, 38(9):755-761.YANG Hu, YUAN Bo, LU Yaobai, et al. Preparation of magnetic PAA/chitosan microspheres and its application in wastewater treatment[J]. Science in China Press,2008,38(9):755-761(in Chinese). [2] 徐雅雯, 徐宏, 丁玮杰, 等. 高Fe3O4含量微米尺寸磁性复合微球的合成及其在化学发光免疫检测中应用初探[J]. 高分子学报, 2010(11):1341-1345.XU Yawen, XU Hong, DING Weijie, et al. Preparation and bio-application of monodisperse composite microspheres with high Fe3O4 content[J]. Acta Polymerica Sinica,2010(11):1341-1345(in Chinese). [3] LIANG X, XI B, XIONG S. Porous soft magnetic material: The maghemite microsphere with hierarchical nanoarchitecture and its application in water purification[J]. Materials Research Bulletin,2009,44(12):2233-2239. doi: 10.1016/j.materresbull.2009.08.003 [4] HATO M J, CHOI H J, SIM H H, et al. Magnetic carbonyl iron suspension with organoclay additive and its magnetorheological properties[J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects,2011,377(1-3):103-109. [5] SEDLACIK M, PAVLINEK V, LEHOCKY M. Plasma-treated carbonyl iron particles as a dispersed phase in magnetorheological fluids[J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects,2011,387(1-3):99-103. [6] LIU Y D, HONG C H, CHOI H J. Polymeric colloidal magnetic composite microspheres and their magneto-responsive characteristics[J]. Macromolecular Research,2012,20(12):1211-1218. doi: 10.1007/s13233-012-0198-8 [7] GOSWAMI M M, DEY C, SARKAR D, et al. Micelles driven magnetite (Fe3O4) hollow spheres and a study on AC magnetic properties for hyperthermia application[J]. Journal of Magnetism & Magnetic Materials,2016,417:376-381. [8] PARK D E, CHAE H S, CHOI H J, et al. Magnetite-polypyrrole core-shell structured microspheres and their dual stimuli-response under electric and magnetic fields[J]. Journal of Materials Chemistry,2015,3(13):3150-3158. [9] 盖青青, 曲锋, 梅芳. 磁性粒子在蛋白质分离纯化中的应用[J]. 化学通报, 2010(2):99-105.GAI Qingqing, QU Feng, MEI Fang. Applications of magnetic particles in proteins separation and purification[J]. Chemical Bulletin,2010(2):99-105(in Chinese). [10] ALVER E, METIN A Ü. Anionic dye removal from aqueous solutions using modified zeolite: Adsorption kinetics and isotherm studies[J]. Chemical Engineering Journal,2012,200-202:59-67. doi: 10.1016/j.cej.2012.06.038 [11] TOOR M, JIN B. Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye[J]. Chemical Engineering Journal,2012,187(2):79-88. [12] PAPAGEORGIOU G Z, BIKIARIS D N. Crystallization and melting behavior of three biodegradable poly(alkylene succinates). A comparative study[J]. Polymer,2005,46(26):12081-12092. doi: 10.1016/j.polymer.2005.10.073 [13] CRINI G, PEINDY H N, GIMBERT F. Basic green 4(malachite green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies[J]. Removal of C: Separation & Purification Technology,2007,53(1):97-110. [14] COTORUELO L M, MARQUÉS M D, DÍAZ F J. Adsorbent ability of lignin-based activated carbons for the removal of p-nitrophenol from aqueous solutions[J]. Chemical Engineering Journal,2012,184(3):176-183. [15] HE Ping, CUI Longlan, QIANG Weili, et al. Preparation and characterization of monodisperse P(St/AA) composite microspheres with high Fe3O4 content[J]. Acta Polymerica Sinica,2007(8):732-736. [16] 余智军, 钱浩, 林志勇, 等. 磁性磺酸阳离子交换树脂微球的制备与表征[J]. 化学工程与设备, 2012(6):8-9.YU Zhijun, QIAN Hao, LIN Zhiyong, et al. Preparation and characterization of magnetic sulfonic acid cation exchange resin microspheres[J]. Chemical Engineering & Equipment,2012(6):8-9(in Chinese). [17] XU Z, XIA A, WANG C, et al. Synthesis of raspberry-like magnetic polystyrene microspheres[J]. Materials Chemistry & Physics,2007,103(2-3):494-499. [18] 邓建国, 贺传兰, 龙新平, 等. 磁性Fe3O4-聚吡咯纳米微球的合成与表征[J]. 高分子学报, 2003(3):393-397. doi: 10.3321/j.issn:1000-3304.2003.03.013DENG Jianguo, HE Chuanlan, LONG Xinping, et al. Preparation and characterization of magnetic Fe3O4-polypyrrole nanoparticles[J]. Acta Polymerica Sinica,2003(3):393-397(in Chinese). doi: 10.3321/j.issn:1000-3304.2003.03.013 [19] SINGH K, OHLAN A, PHAM V H. Nanostructured graphene/Fe3O4 incorporated polyaniline as a high performance shield against electromagnetic pollution[J]. Nanoscale,2013,5(6):2411-2420. doi: 10.1039/c3nr33962a [20] MOHAMMADI A, BARIKANI M, LAKOURAJ M M. Biocompatible polyurethane/thiacalix[4] arenes functionalized Fe3O4 magnetic nanocomposites: Synthesis and properties[J]. Materials Science & Engineering C,2016,66:106-118. -





 下载:
下载: