Application of photocatalytic degradation of formaldehyde by g-C3N4-Ag/SiO2 heterostructure composites
-
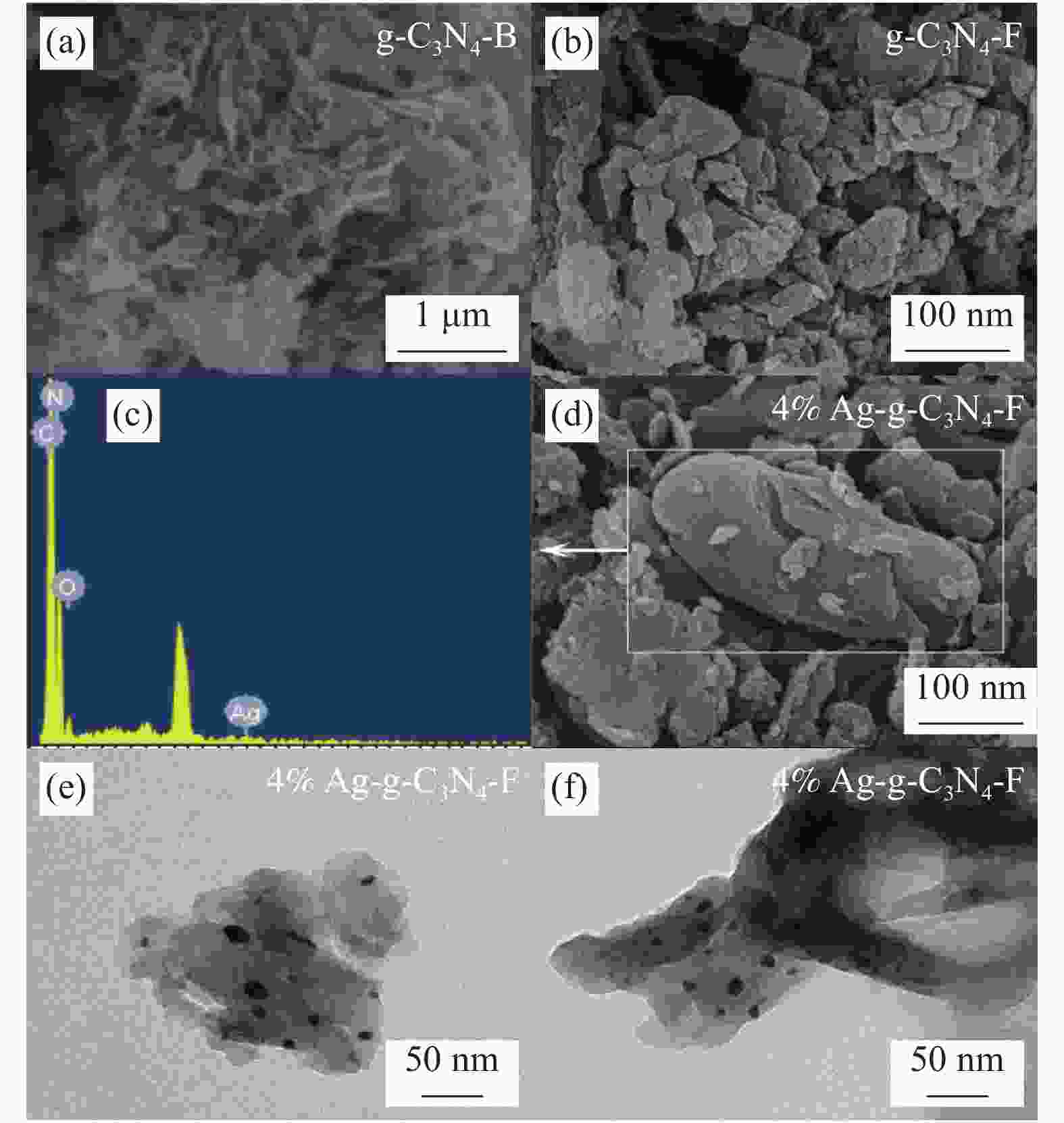
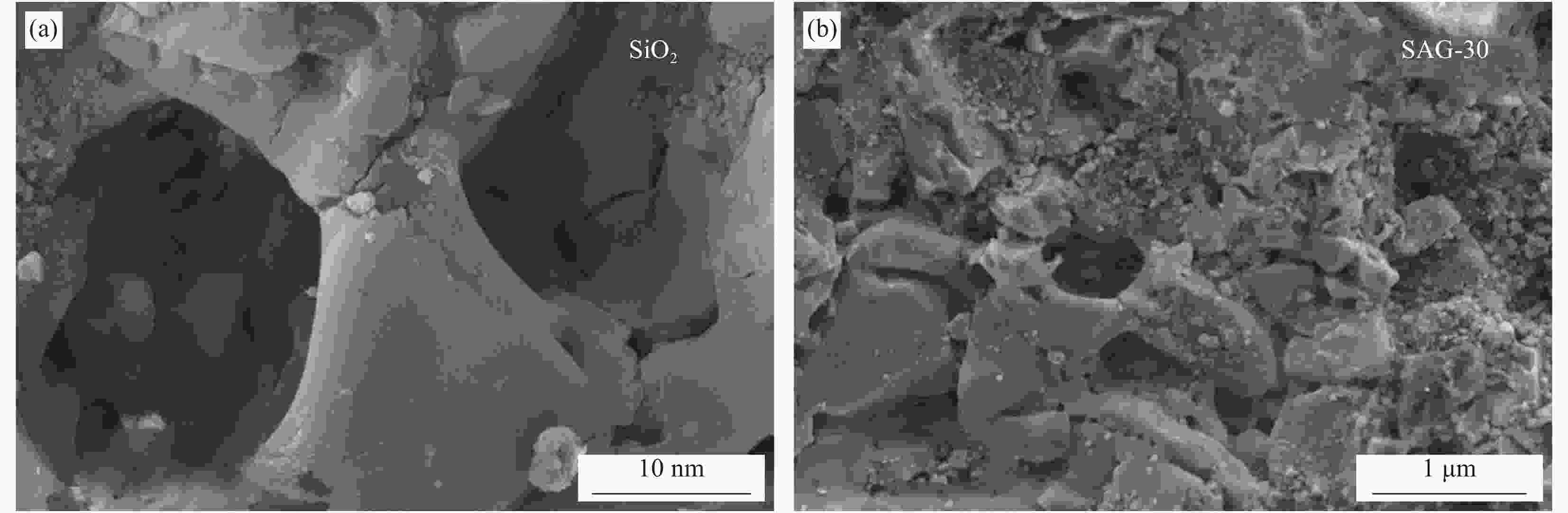
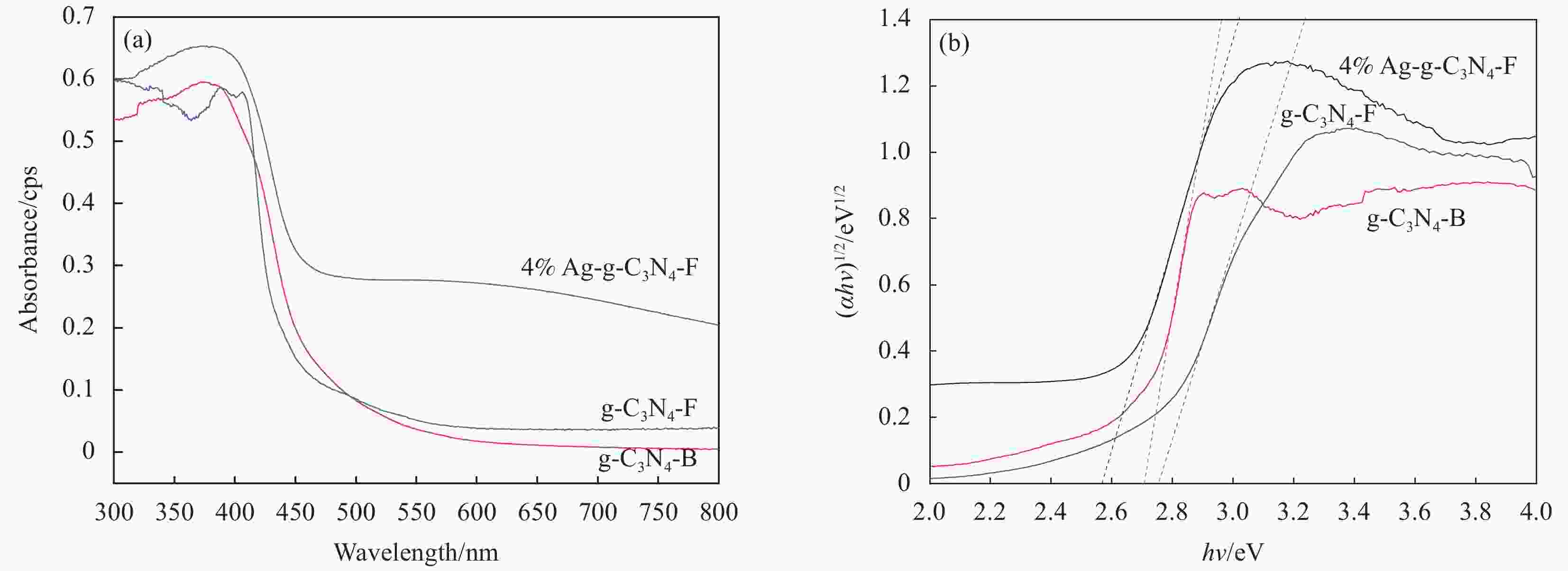
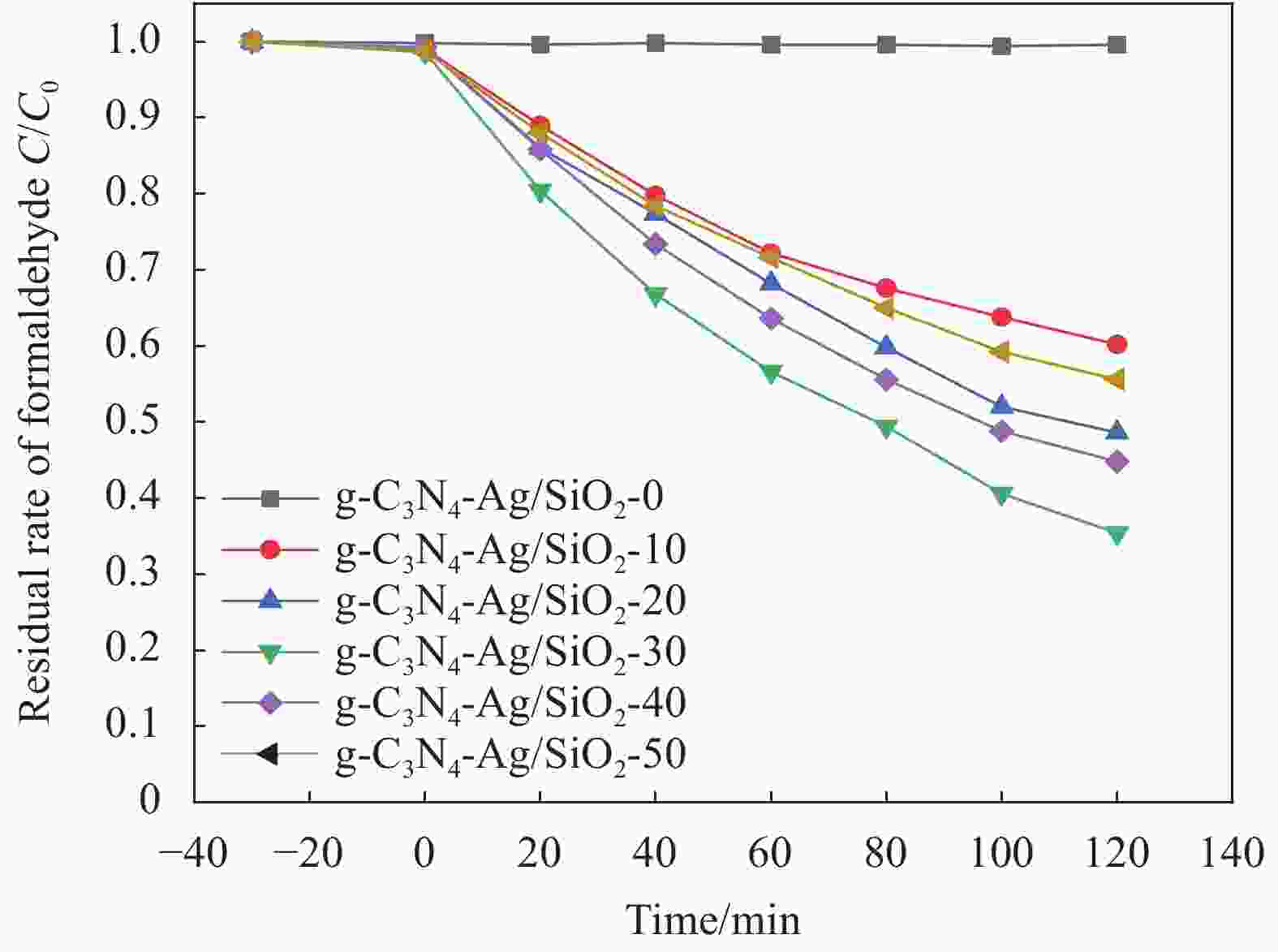
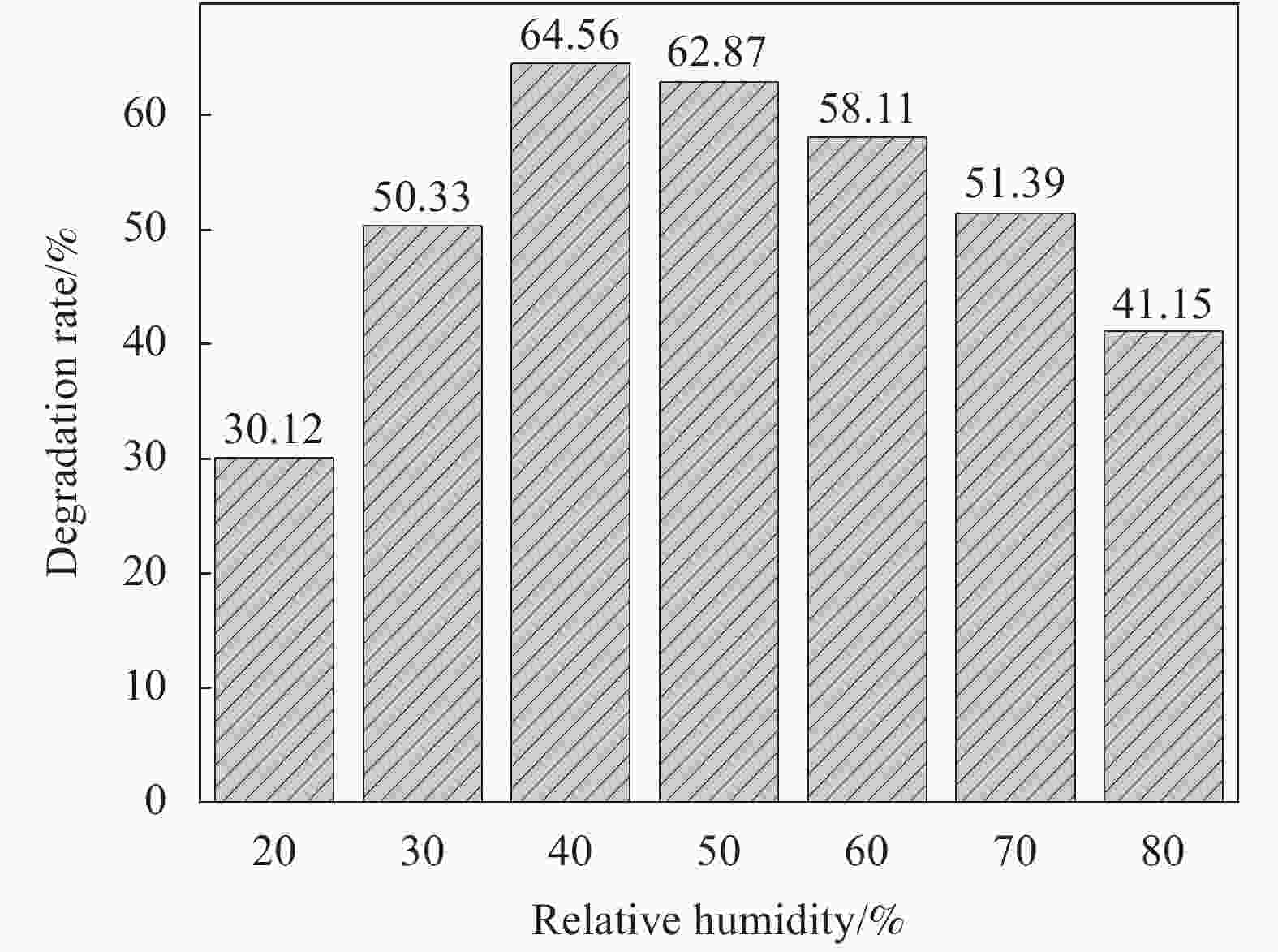
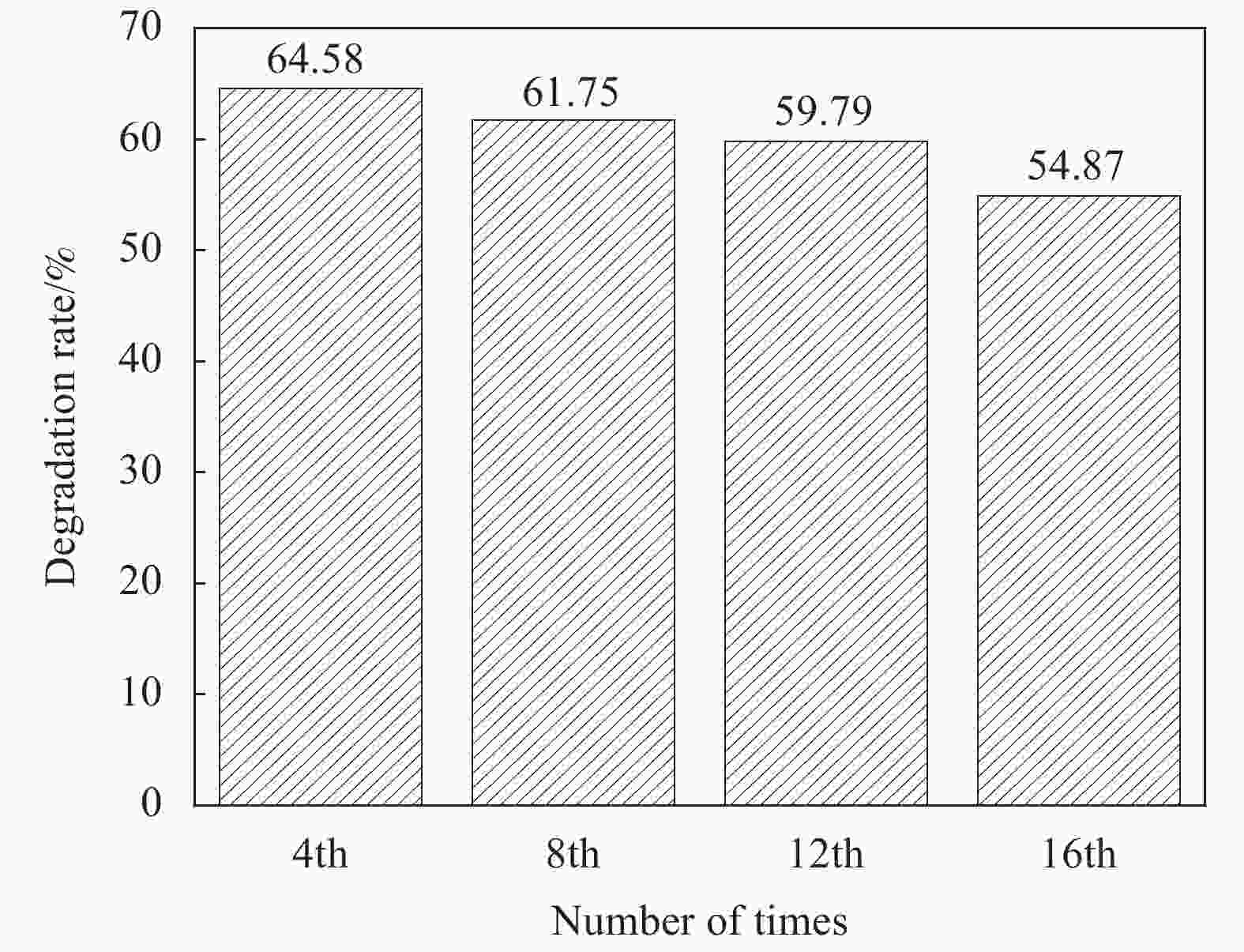
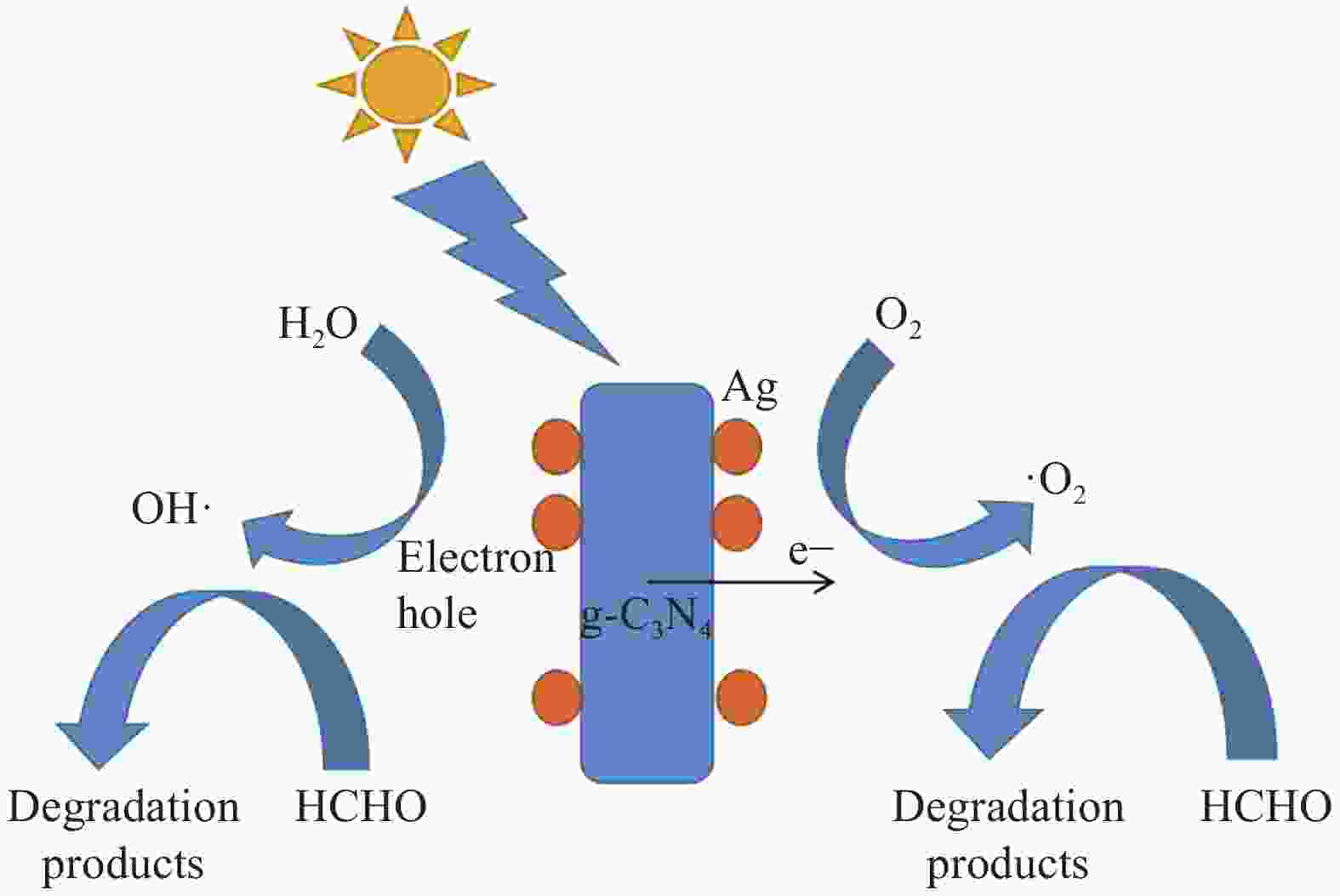
摘要: 将Ag、薄层石墨相氮化碳(g-C3N4)和硅铝胶球(SiO2)通过液相超声剥离-光化学沉积法-浸渍法合成复合光催化材料。设计甲醛降解密闭实验舱,探究g-C3N4、Ag-g-C3N4和g-C3N4-Ag/SiO2材料的光催化特性及其对甲醛的降解效果。结果表明,在可见光源条件下,对于g-C3N4-Ag/SiO2材料,降解甲醛的效率最高可达到65.6%。40%的相对湿度可有效提升降解效果。负载30 mg 4%Ag/g-C3N4的硅铝胶球循环使用16次时,甲醛降解效率仅下降9.71%。结合材料表征结果表明,通过超声剥离和Ag的引入,提升了材料可见光的吸收强度和吸收范围,并且有效促进了光生电子和空穴的分离,有效提升甲醛分子的降解效率。研究结果表明g-C3N4-Ag/SiO2材料具有优异的稳定性和良好的光催化性能,为实际有机污染物治理的应用提供了较好的科学基础。Abstract: Composite photocatalytic materials were synthesized by liquid-phase ultrasonic exfoliation-photochemical deposition method-impregnation method of monolithic Ag, thin layer of graphitic phase carbon nitride (g-C3N4) and silica-alumina colloidal spheres (SiO2). A formaldehyde degradation hermetic chamber was designed to investigate the photocatalytic properties of g-C3N4, Ag-g-C3N4 and g-C3N4-Ag/SiO2 materials and their degradation effects on formaldehyde. The results show that the efficiency of degradation of formaldehyde can reach up to 65.6% for SAG materials under visible light source conditions. 40% relative humidity can effectively enhance the degradation efficiency.The formaldehyde degradation efficiency decreases by only 9.71% when the silica-alumina spheres load with 30 mg 4%Ag/g-C3N4 are recycled for 16 times. The results of material characterization show that the visible light absorption intensity and absorption range of the material are enhanced by ultrasonic stripping and the introduction of Ag, and the separation of photogenerated electrons and holes are effectively promoted, which effectively enhance the degradation efficiency of formaldehyde molecules. The results show that the g-C3N4-Ag/SiO2 material has excellent stability and good photocatalytic performance, which provides a better scientific basis for the application of practical organic pollutant treatment.
-
Key words:
- silica-alumina colloidal spheres /
- Ag /
- photocatalysis /
- graphitic phase carbon nitride /
- formaldehyde /
- degradation
-
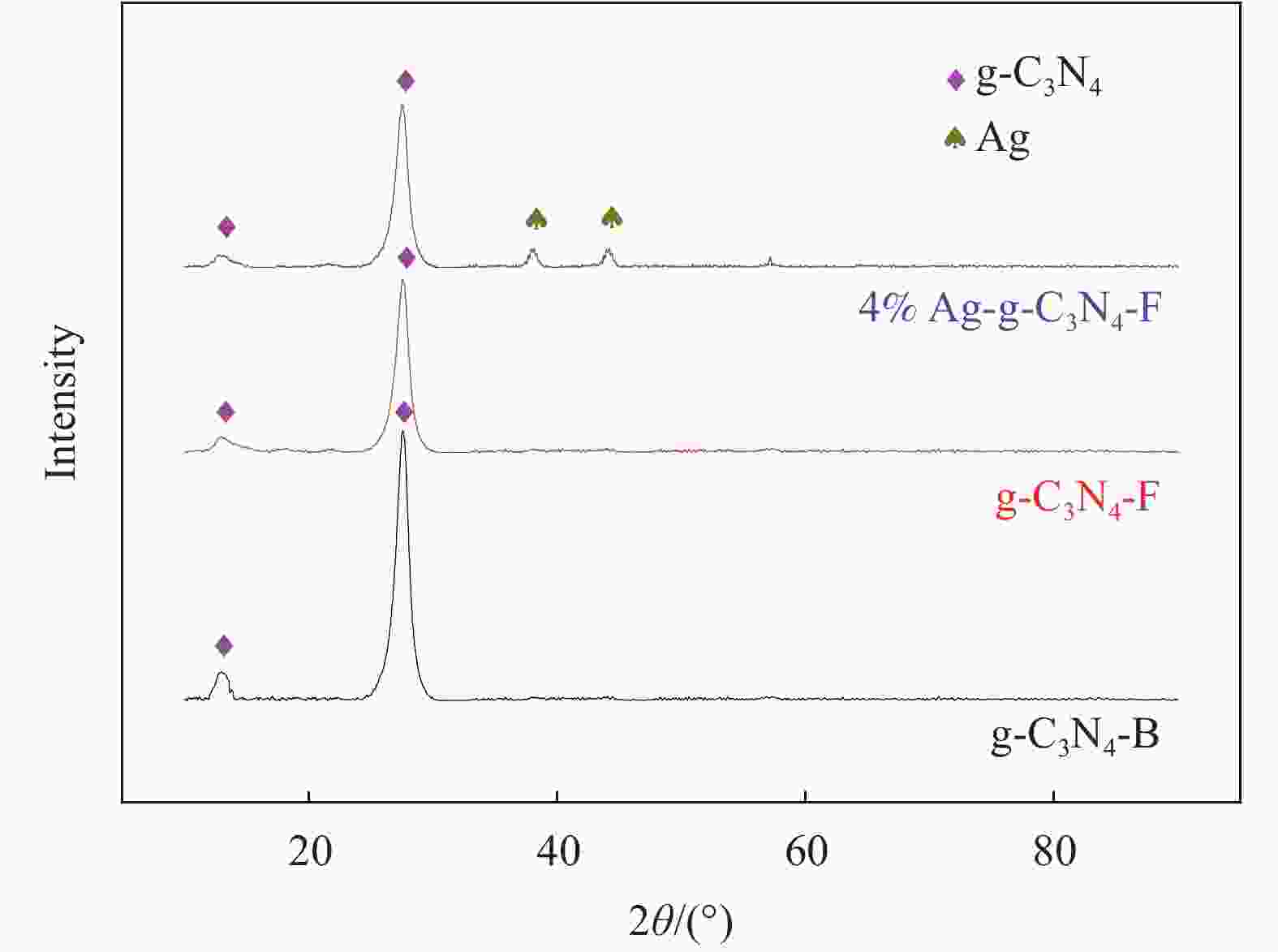
表 1 Ag-薄层石墨相氮化碳(g-C3N4-F)复合材料的命名
Table 1. Naming of Ag-thin layer graphite phase carbon nitride (g-C3N4-F) composites
Sample g-C3N4-F/mg Concentration of AgNO3/(mg·mL−1) AgNO3/mL Na2S/mL 0 500 0 5 2 1%Ag-g-C3N4-F 500 1 5 2 2%Ag-g-C3N4-F 500 2 5 2 4%Ag-g-C3N4-F 500 4 5 2 8%Ag-g-C3N4-F 500 8 5 2 12%Ag-g-C3N4-F 500 12 5 2 16%Ag-g-C3N4-F 500 16 5 2 表 2 石墨相氮化碳(g-C3N4)-Ag/SiO2复合材料的命名
Table 2. Naming of graphite phase carbon nitride (g-C3N4)-Ag/SiO2 composites
Sample Ag-g-C3N4-F/mg SiO2/g Ethyl alcohol/mL g-C3N4-Ag/SiO2-10 10 30 100 g-C3N4-Ag/SiO2-20 20 30 100 g-C3N4-Ag/SiO2-30 30 30 100 g-C3N4-Ag/SiO2-40 40 30 100 g-C3N4-Ag/SiO2-50 50 30 100 -
[1] ABU J B, MOURAD A H, HITTINI W, et al. Traditional, state-of-the-art and renewable thermal building insulation materials: An overview[J]. Construction and Building Materials,2019,214:709-735. [2] HUANG S, WEI W, WESCHLER L B, et al. Indoor formaldehyde concentrations in urban China: Preliminary study of some important influencing factors[J]. Science of the Total Environment,2017,590:394-405. [3] WI S, PARK J H, KIM Y U, et al. Evaluation of environmental impact on the formaldehyde emission and flame-retardant performance of thermal insulation materials[J]. Journal of Hazardous Materials,2020,402:123463. [4] XIAN Q X, YING Y Y, YI T N, et al. Development of a cornstarch adhesive for laminated veneer lumber bonding for use in engineered wood flooring[J]. International Journal of Adhesion and Adhesives,2019,98:102534. [5] 尚丽新, 朴丰源. 环境有害因素的生殖和发育毒性[M]. 郑州: 河南科学技术出版社, 2017: 1-312.SHANG L X, PU F Y, ed. Reproductive and developmental toxicity of environmental harmful factors[M]. Zhengzhou: Henan Science and Technology Press, 2017: 1-312(in Chinese). [6] IARC. IARC monographs on the evaluation of carcinogenic risks to humans[R]. Switzerland:World Health Organization, 2006, 95: 27-50. [7] CHAE J N, MI J, DANIEL C W, et al. High-performance materials for effective sorptive removal of formaldehyde in air[J]. Journal of Hazardous Materials,2018,27(2):442-470. [8] KUAN L P, DAI L C, GUAN T P, et al. Removal of phenol from gas streams via combined plasma catalysis[J]. Journal of Industrial and Engineering Chemistry,2017,52:108-120. doi: 10.1016/j.jiec.2017.03.031 [9] PATRICIO A, MORENO C, FELIPE S, et al. Computational tomography and CFD simulation of a biofilter treating a toluene, formaldehyde and benzo[α]pyrene vapor mixture[J]. Chemosphere,2020,240:124924. doi: 10.1016/j.chemosphere.2019.124924 [10] ISMAEL M, WU Y. A mini-review on the synthesis and structural modification of g-C3N4 -based materials, and their applications in solar energy conversion and environmental remediation[J]. Sustainable Energy & Fuels,2019,3(12):8946-8968. [11] LI Y, WU S, HUANG L, et al. Synthesis of carbon-doped g-C3N4 composites with enhanced visible-light photocatalytic activity[J]. Materials Letters,2014,137:281-284. doi: 10.1016/j.matlet.2014.08.142 [12] WANG X, MAEDA K, CHEN X, et al. Polymer semiconductors for artificial photosynthesis: Hydrogen evolution by mesoporous graphitic carbon nitride with visible light[J]. Journal of the American Chemical Society,2009,131(5):1680-1681. doi: 10.1021/ja809307s [13] ZHOU T, XU Y G, XU H. In situ oxidation synthesis of visible-lightdriven plasmonic photocatalyst Ag/AgCl/g-C3N4 and its activity[J]. Ceramics International,2014,40(7):9293-9301. doi: 10.1016/j.ceramint.2014.01.152 [14] LIANG K, ZHANG C H, XIANG H W, et al. Effects of modified SiO2 on H2 and CO adsorption and hydrogenation of iron-based catalysts[J]. Journal of Fuel Chemistry and Technology,2019,47(7):769-779. doi: 10.1016/S1872-5813(19)30033-7 [15] WAN J W, YU J C, DEHUA X, et al. Graphene and g-C3N4 nanosheets cowrapped elemental α-sulfur as a novel metal-free heterojunction photocatalyst for bacterial inactivation under visible-light[J]. Environmental Science & Technology,2013,47(15):8724-8732. [16] WANG X C, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials,2009,8(1):76-80. doi: 10.1038/nmat2317 [17] LIU L, QI Y, YANG J, et al. An AgI@ g-C3N4 hybrid core@shell structure: stable and enhanced photocatalytic degradation[J]. Applied Surface Science,2015,358(A):319-327. [18] CHENG B, LE Y, YU J. Preparation and enhanced photocatalytic activity of Ag@TiO2 core-shell nanocomposite nanowires[J]. Journal of Hazardous Materials,2010,177(1-3):971-977. doi: 10.1016/j.jhazmat.2010.01.013 [19] JI H, CHANG F, HU X, et al. Photocatalytic degradation of 2, 4, 6-trichlorophenol over g-C3N4 under visible light irradiation[J]. Chemical Engineering Journal,2013,218:183-190. doi: 10.1016/j.cej.2012.12.033 [20] CAO S W, YUAN Y P, FANG J, et al. In-situ growth of CdS quantum dots on g-C3N4 nanosheets for highly efficient photocatalytic hydrogen generation under visible light irradiation[J]. International Journal of Hydrogen Energy,2013,38(3):1258-1266. doi: 10.1016/j.ijhydene.2012.10.116 [21] CHEN Y, HUANG W, HE D, et al. Construction of heterostructured g-C3N4/Ag/TiO2 microspheres with enhanced photocatalysis performance under visible-light irradiation[J]. ACS Applied Materials & Interfaces,2014,6(16):17523-17539. [22] ZHOU C, SHI R, SHANG L, et al. Template-free large-scale synthesis of g-C3N4 microtubes for enhanced visible light-driven photocatalytic H2 production[J]. Nano Research,2018,11(6):3462-3468. doi: 10.1007/s12274-018-2003-2 [23] SHI H, CHEN G, ZHANG C, et al. Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel[J]. Acs Catalysis,2014,4(10):3637-3643. doi: 10.1021/cs500848f [24] WEN X J, NIU C G, ZHANG L, et al. In-situ synthesis of visible-light-driven plasmonic Ag/AgCl-CdWO4 photocatalyst[J]. Ceramics International,2016,43(2):1922-1929. [25] JO B W, KIM C H, TAE G H, et al. Characteristics of cement mortar with nano-SiO2 particles[J]. Construction and Building Materials,2007,21(6):1351-1355. doi: 10.1016/j.conbuildmat.2005.12.020 [26] SUN Y, YANG J, YANG S, et al. Development of an immunochromatographic lateral flow strip for the simultaneous detection of aminoglycoside residues in milk[J]. Rsc Advances,2018,8(17):9580-9586. doi: 10.1039/C8RA01116H -





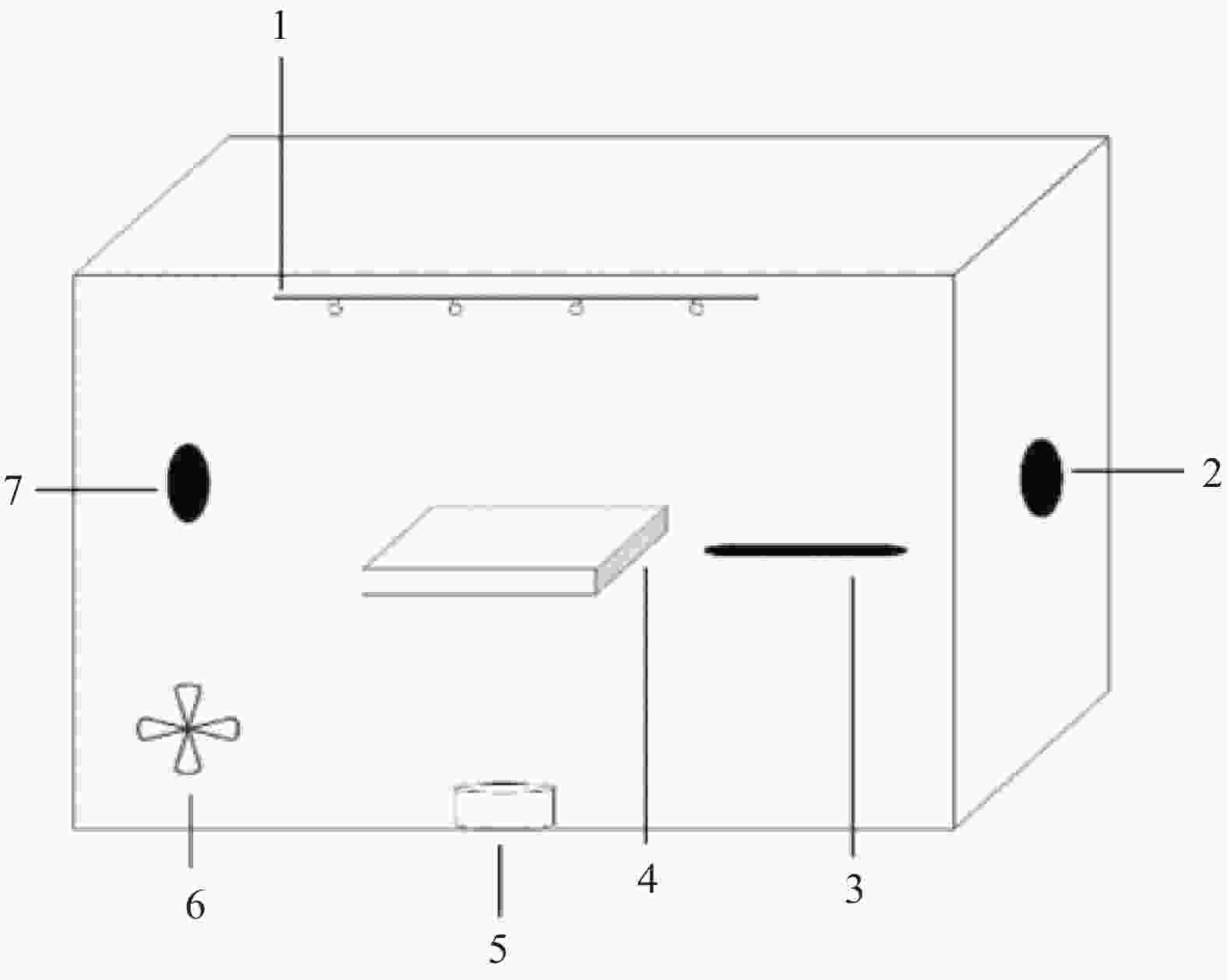
 下载:
下载: