Preparation and electrochemical properties of Li-Ni co-doping spinel LiMn2O4 single crystal polyhedron material
-
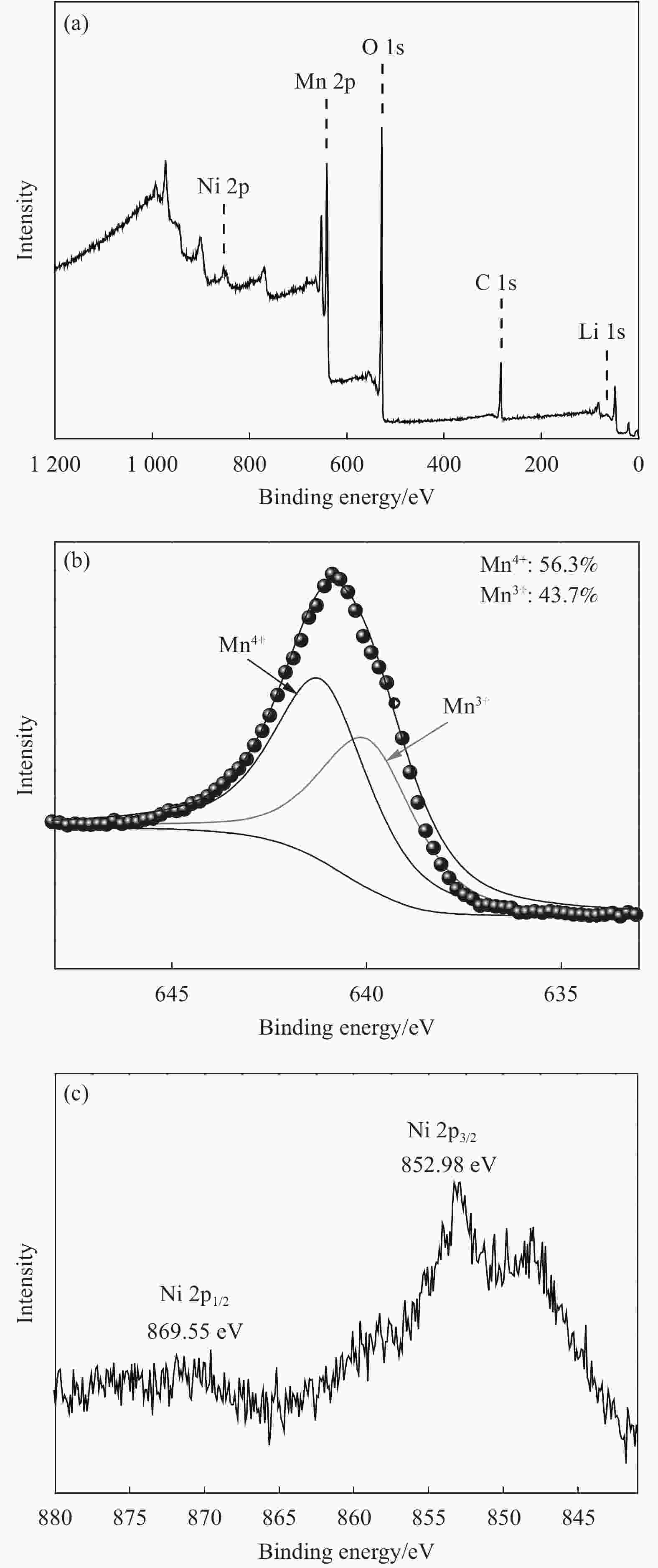
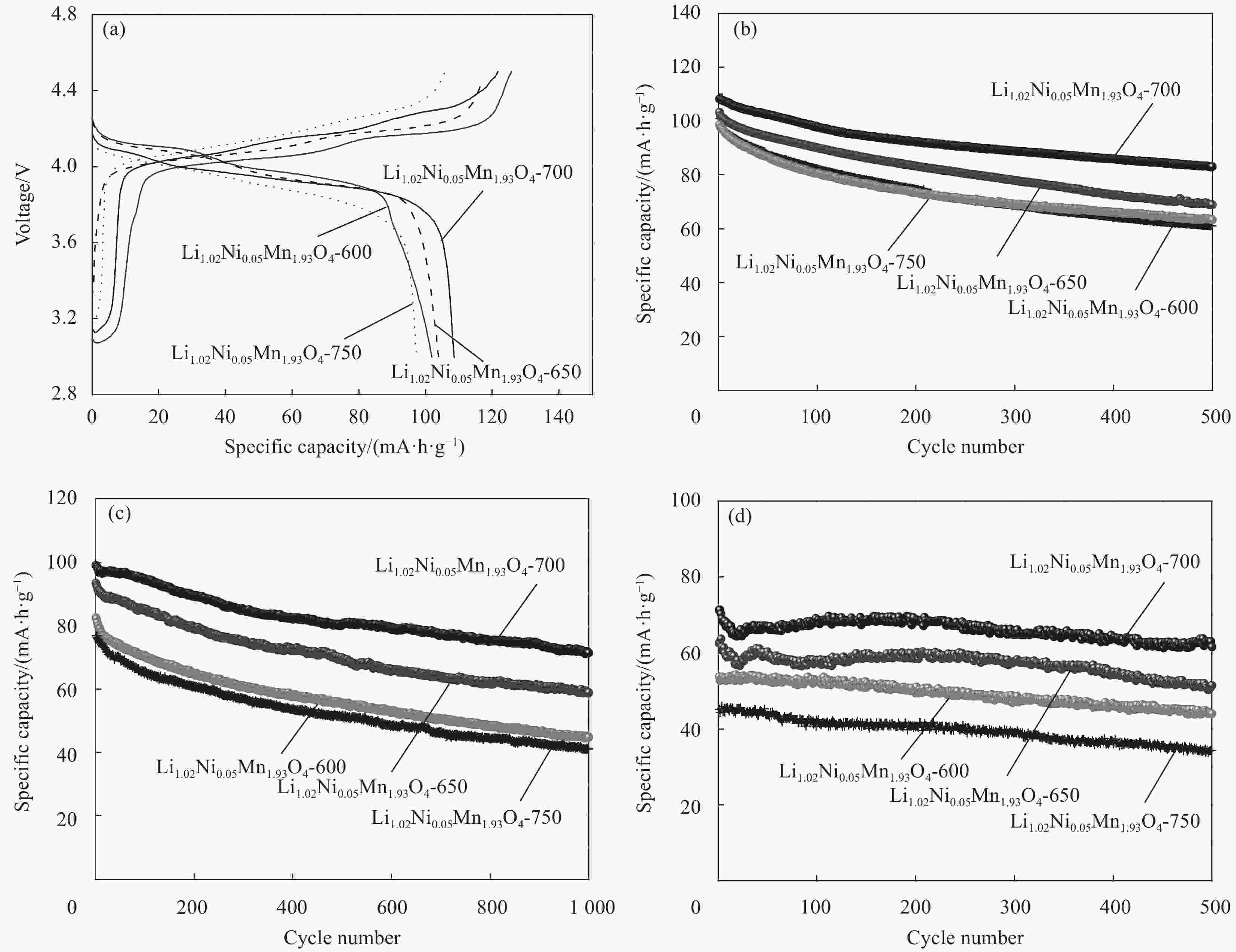
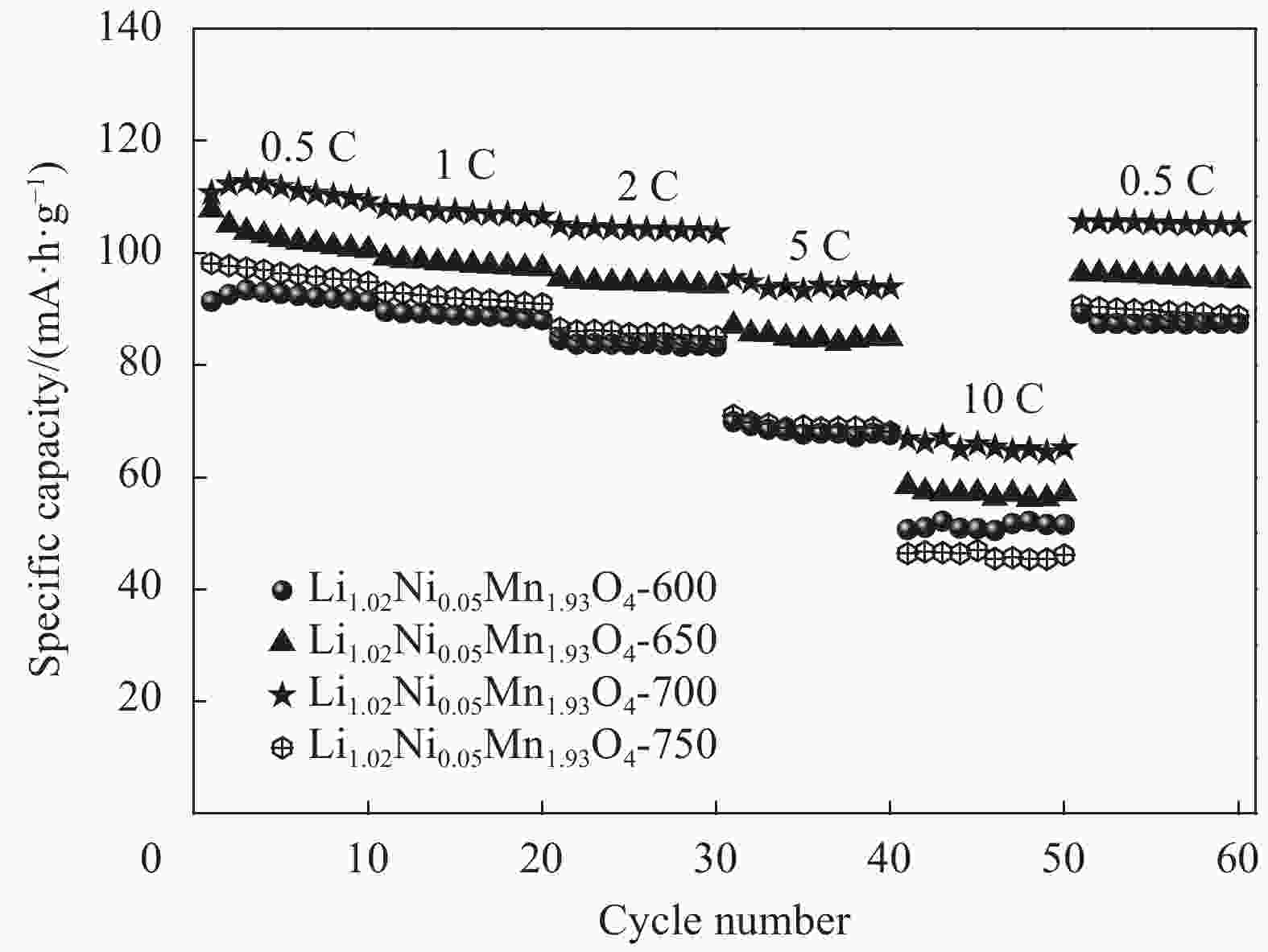
摘要: 采用无焰燃烧法在500℃反应3 h,然后分别在600、650、700和750℃二次焙烧6 h制备了尖晶石型Li1.02Ni0.05Mn1.93O4正极材料。结果表明,不同焙烧温度制备的Li-Ni共掺材料没有改变LiMn2O4的立方尖晶石结构,且随着焙烧温度的升高,颗粒尺寸变大,结晶性提高。二次焙烧温度为700℃的Li1.02Ni0.05Mn1.93O4单晶多面体晶粒正极材料具有{111}、{110}和{100}面,且电化学性能较优,在1 C倍率下初始放电比容量为108.2 mA·h·g−1,循环500次后的容量保持率为76.8%;在5 C下首次放电比容量可达到99.0 mA·h·g−1,1000次循环后,仍能维持72.1%的容量保持率;在10 C下仍显示出71.3 mA·h·g−1的首次放电比容量及经500次循环后86.4%的容量保持率。并且其具有较大的Li+扩散系数和较小的表观活化能。Li-Ni共掺LiMn2O4单晶多面体材料能够有效抑制Jahn-Teller效应,减小Mn的溶解及增加Li+扩散通道,使材料的晶体结构稳定,提高倍率性能和循环性能。Abstract: Spinel Li1.02Ni0.05Mn1.93O4 cathode material was synthesized via a flameless combustion method at 500℃ for 3 h followed by calcination at 600、650、700 and 750℃ for 6 h, respectively. The results show that Li-Ni co-doping material at different calcination temperatures does not change the cubic spinel structure of LiMn2O4. With the increase of calcination temperature, the particle size increases and the crystallinity is enhanced. At the secondary calcination temperature of 700℃, the cathode material of Li1.02Ni0.05Mn1.93O4 single crystal polyhedron possess {111}, {110} and {100} surfaces. It exhibits an excellent electrochemical performance. The Li1.02Ni0.05Mn1.93O4 cathode material shows the first-discharge specific capacity of 108.2 mA·h·g−1 and maintains the capacity retention of 76.8% after 500 cycles at 1 C. Moreover, it displays an initial discharge capacity of 99.0 and 71.3 mA·h·g−1, while provides a capacity retention rate of 72.1 and 86.4% after 1000 cycles at 5 C and 500 cycles at 10 C, respectively. The optimized electrode has a large Li+ diffusion coefficient and low apparent activation energy. The Li-Ni co-doping material of LiMn2O4 single crystal polyhedron can inhibit the Jahn-Teller effect effectively and alleviate Mn dissolution as well as increase Li+ ions expressing channels. Hence, the crystal structure of material is stabilized, the rate and cycle performance are enhanced.
-
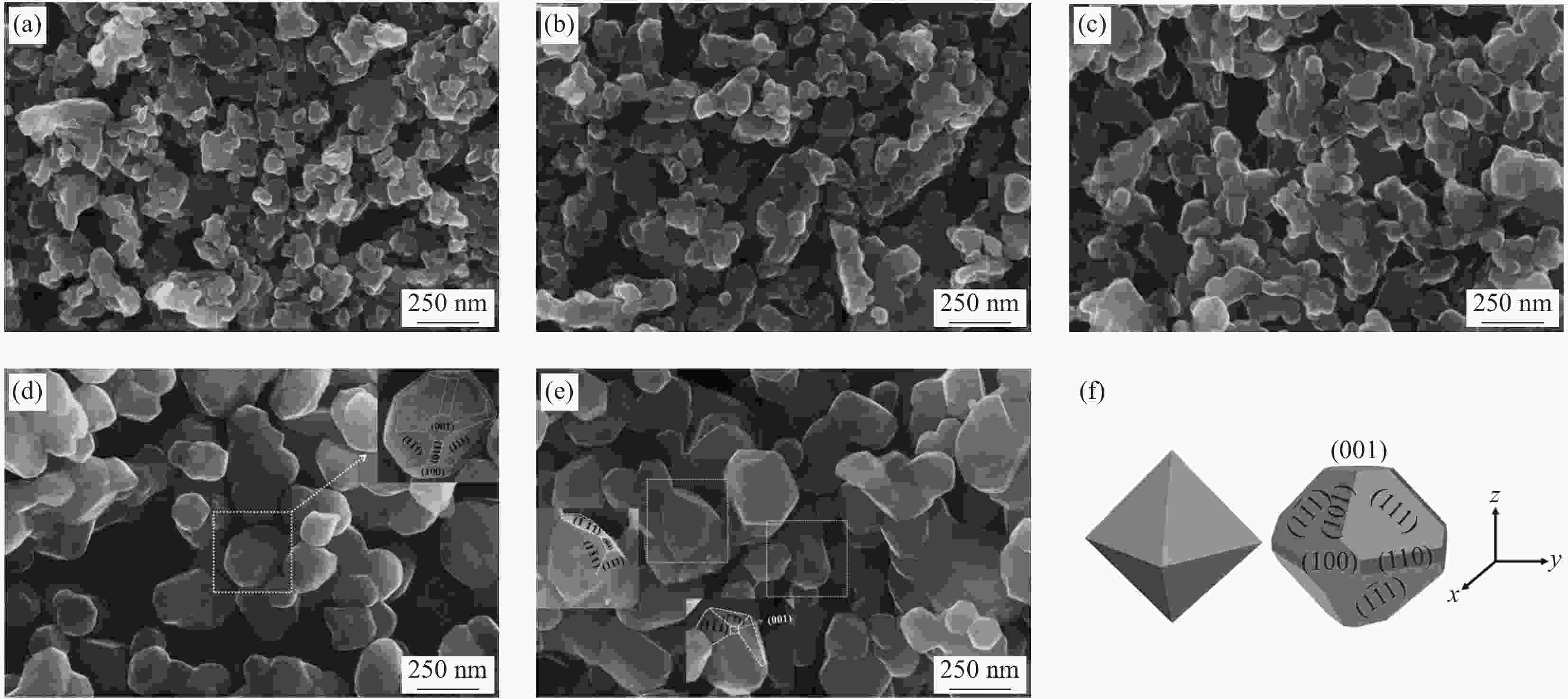
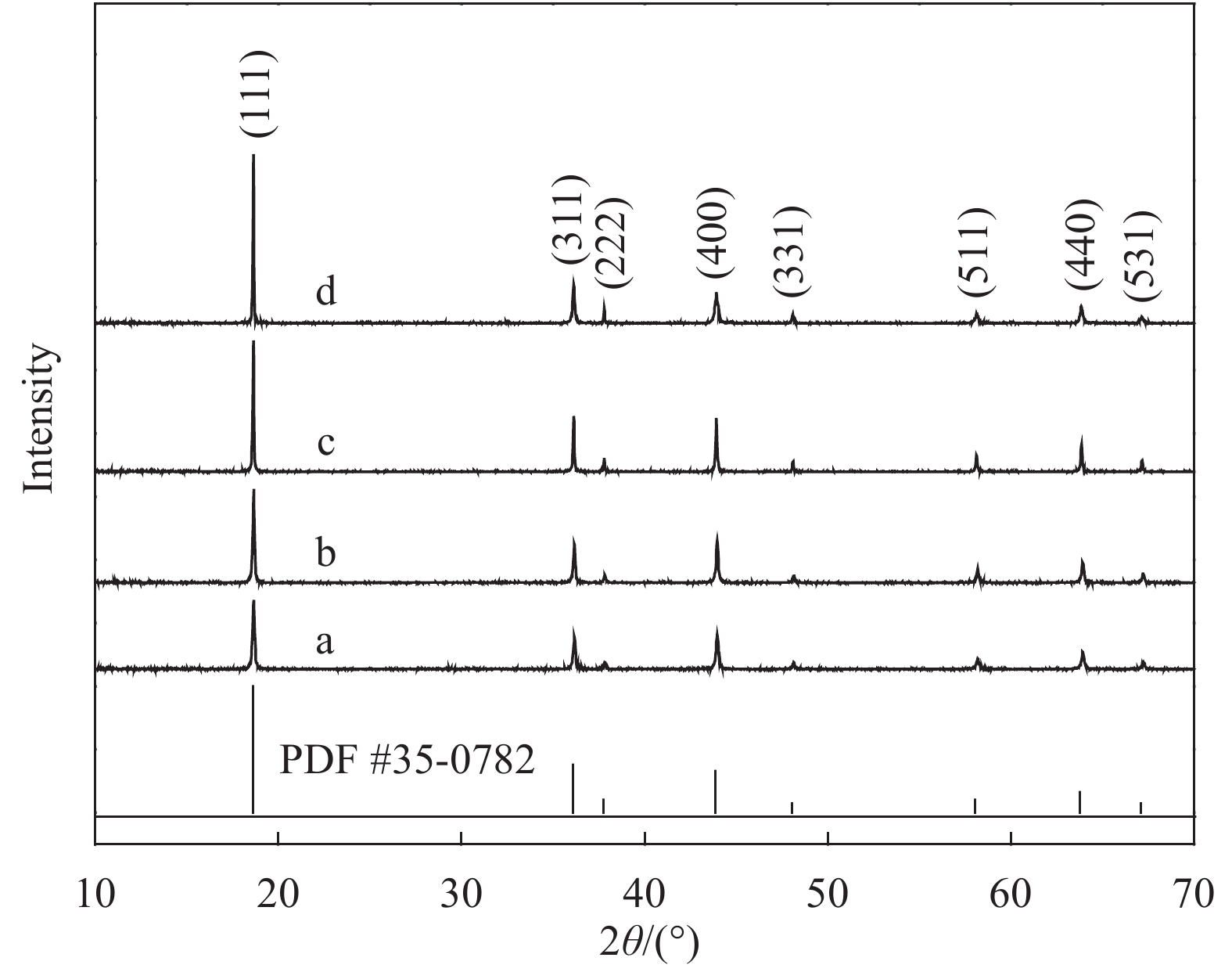
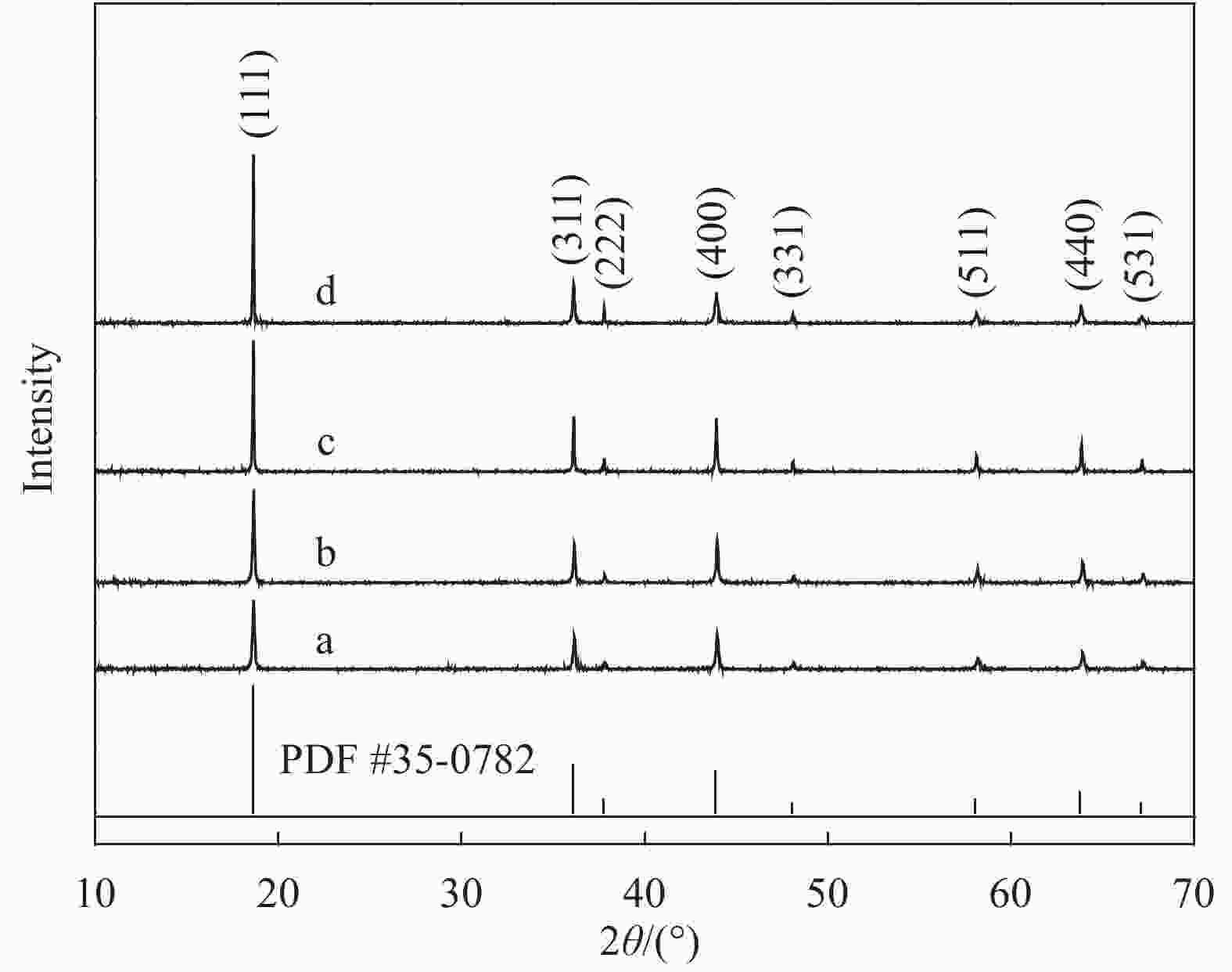
图 2 Li1.02Ni0.05Mn1.93O4-x样品的SEM图像:(a) 500℃燃烧反应3 h产物;(b) Li1.02Ni0.05Mn1.93O4-600;(c) Li1.02Ni0.05Mn1.93O4-650;(d) Li1.02Ni0.05Mn1.93O4-700;(e) Li1.02Ni0.05Mn1.93O4-750;(f) 八面体及多面体示意图
Figure 2. SEM images of Li1.02Ni0.05Mn1.93O4-x: (a) Combustion at 500℃ for 3 h; (b) Li1.02Ni0.05Mn1.93O4-600; (c) Li1.02Ni0.05Mn1.93O4-650; (d) Li1.02Ni0.05Mn1.93O4-700; (e) Li1.02Ni0.05Mn1.93O4-750; (f) Schematic diagram of octahedron and polyhedron
表 1 样品的命名
Table 1. Naming of samples
Sample Secondary calcination temperature/℃ Li1.02Ni0.05Mn1.93O4-600 600 Li1.02Ni0.05Mn1.93O4-650 650 Li1.02Ni0.05Mn1.93O4-700 700 Li1.02Ni0.05Mn1.93O4-750 750 表 2 不同二次焙烧温度Li1.02Ni0.05Mn1.93O4-x样品在循环前和500次循环后的电荷转移阻抗Rct
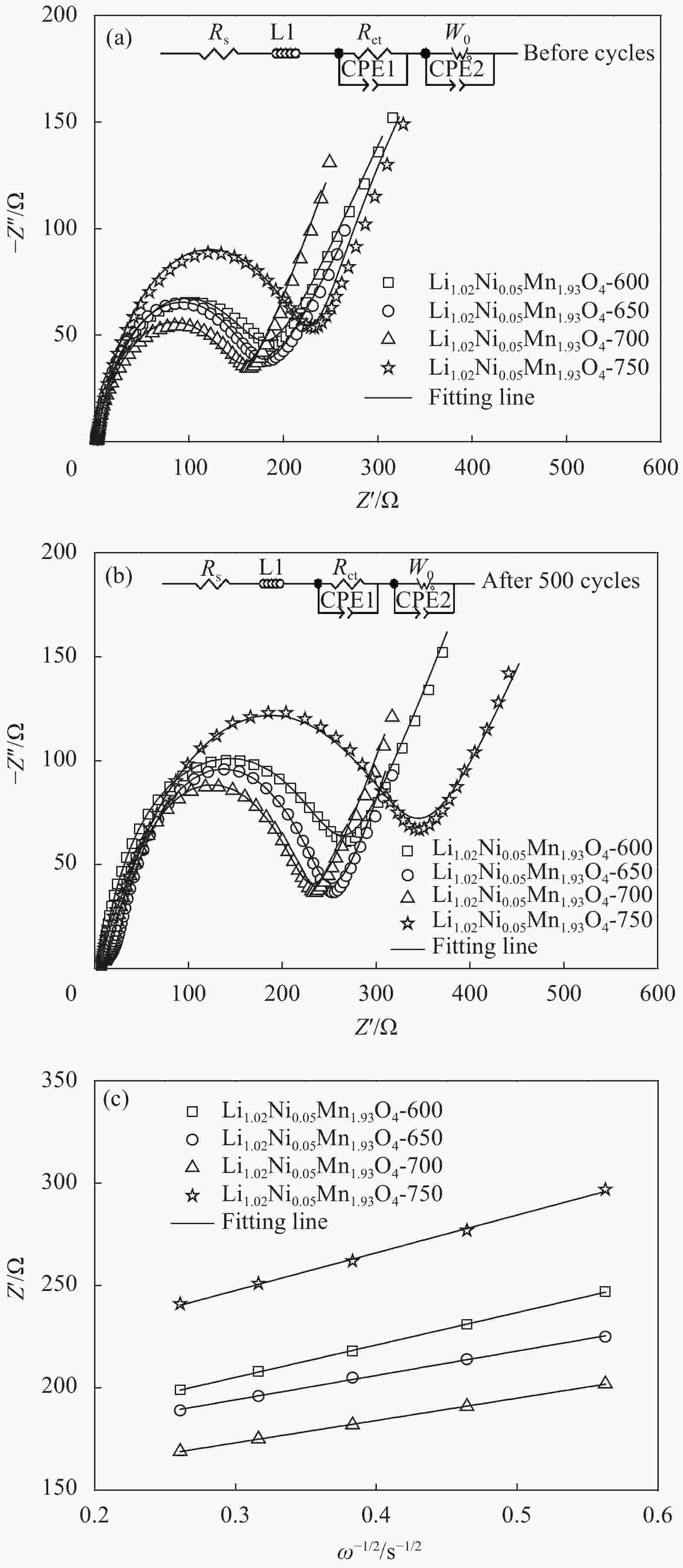
Table 2. Charge transfer impedance Rct of Li1.02Ni0.05Mn1.93O4-x at different secondary calcination temperatures before and after 500 cycles
Sample Before cycles After 500 cycles Rs/Ω Rct/Ω Rs/Ω Rct/Ω Li1.02Ni0.05Mn1.93O4-600 4.02 184.98 7.31 260.69 Li1.02Ni0.05Mn1.93O4-650 2.78 178.22 10.40 242.60 Li1.02Ni0.05Mn1.93O4-700 2.63 160.37 7.92 225.08 Li1.02Ni0.05Mn1.93O4-750 3.18 229.82 14.70 331.30 Note: Rs—Electrolyte impedanc. -
[1] NAOKI N, WU F X, LEE J T, et al. Li-ion battery materials: Present and future[J]. Materials Today,2015,18(5):252-264. [2] PARK O K, CHO Y, LEE S, et al. Who will drive electric vehicles, olivine or spinel[J]. Energy & Environmental Science,2011,4(5):1621-1633. [3] 李军, 李少芳, 李庆彪, 等. Cr3+掺杂LiNi0.5Mn1.5O4材料的制备及性能[J]. 稀有金属材料与工程, 2017, 46(11):3458-3463.LI Jun, LI Shaofang, LI Qingbiao, et al. Synthesis and performance study of Cr-doped LiNi0.5Mn1.5O4 cathode material[J]. Rare Metal Materials and Engineering,2017,46(11):3458-3463(in Chinese). [4] 饶帆, 陈爱华, 赵永彬. 锂离子电池正极材料LiNi0.8Co0.15Al0.05O2的制备与性能[J]. 复合材料学报, 2018, 35(4):946-956.RAO Fan, CHEN Aihua, ZHAO Yongbin. Preparation and performance of LiNi0.8Co0.15Al0.05O2 cathode material of lithium ion battery[J]. Acta Materiae Compositae Sinica,2018,35(4):946-956(in Chinese). [5] JIANG C H, TANG Z L, WANG S T, et al. A truncated octahedral spinel LiMn2O4 as high-performance cathode material for ultrafast and long-life lithium-ion batteries[J]. Journal of Power Sources,2017,357(31):144-148. [6] CAI Y J, HUANG Y D, WANG X C, et al. Long cycle life, high rate capability of truncated octahedral LiMn2O4 cathode materials synthesized by a solid-state combustion reaction for lithium ion batteries[J]. Ceramics International,2014,40(9):14039-14043. [7] TANG X, LIN B H, GE Y, et al. LiMn2O4 nanorod arrays: A potential three-dimensional cathode for lithium-ion microbatteries[J]. Materials Research Bulletin,2015,69:2-6. [8] LIU Y Z, ZHANG M H, XIA Y G, et al. One-step hydrothermal method synthesis of core-shell LiNi0.5Mn1.5O4 spinel cathodes for Li-ion batteries[J]. Journal of Power Sources,2014,256:66-71. [9] CHO J, KIM T J, KIM Y J, et al. Complete blocking of Mn3+ ion dissolution from a LiMn2O4 spinel intercalation compound by Co3O4 coating[J]. Chemical Communications,2001(12):1074-1075. [10] RAGAVENDRAN K, XIA H, MANDAL P, et al. Jahn-Teller effect in LiMn2O4: Influence on charge ordering, magnetoresistance and battery performance[J]. Physical Chemistry Chemical Physics,2017,19(3):2073-2077. [11] DENG B H, NAKAMURA H, YOSHIO M. Capacity fading with oxygen loss for manganese spinels upon cycling at elevated temperatures[J]. Journal of Power Sources,2008,180(2):864-868. [12] WEN W C, JU B W, WANG X Y, et al. Effects of magnesium and fluorine co-doping on the structural and electrochemical performance of the spinel LiMn2O4 cathode materials[J]. Electrochimica Acta,2014,147:271-278. [13] KIM J S, KIM K S, CHO W, et al. A truncated manganese spinel cathode for excellent power and lifetime in lithium-ion batteries[J]. Nano Letters,2012,12(12):6358-6365. [14] 伊廷锋, 胡信国, 高昆, 等. 影响锂离子电池正极材料LiMn2O4性能的因素[J]. 稀有金属快报, 2005, 24(12):1-5.YI Tingfeng, HU Xinguo, GAO Kun, et al. Factors influencing performance of positive materials LiMn2O4 for lithium ion battery[J]. Rare Metals Express,2005,24(12):1-5(in Chinese). [15] PERAMUNAGE D, ABRAHOM K M. Preparation and electrochemical characterization of overlithiated spinel LiMn2O4[J]. Journal of The Electrochemical Society,1998,145(4):1131-1136. [16] SHU J, YI T F, SHUI M, et al. Comparison of electronic property and structural stability of LiMn2O4 and LiNi0.5Mn1.5O4 as cathode materials for lithium-ion batteries[J]. Computational Materials Science,2010,50(2):776-779. [17] 秦毅红, 马尚德, 张云河. 锂镍复合掺杂尖晶石LiMn2O4的制备及电化学性能[J]. 电源技术, 2008, 132(5):293-295. doi: 10.3969/j.issn.1002-087X.2008.05.004QIN Yihong, MA Shangde, ZHANG Yunhe. Preparation and electrochemical property of Li and Ni co-doped LiMn2O4[J]. Chinese Journal of Power Sources,2008,132(5):293-295(in Chinese). doi: 10.3969/j.issn.1002-087X.2008.05.004 [18] HWANG B J, SANTHANAM R, HU S G. Synthesis and characterization of multidoped lithium manganese oxide spinel, Li1.02Co0.1Ni0.1Mn1.8O4, for rechargeable lithium batteries[J]. Journal of Power Sources,2002,108(1-2):250-255. [19] HENDRIKS R, CUNHA D M, SINGH D P, et al. Enhanced lithium transport by control of crystal orientation in spinel LiMn2O4 thin film cathodes[J]. ACS Applied Energy Materials,2018,1(12):7046-7051. [20] 于月, 白红丽, 刘晓芳, 等. 液相无焰燃烧合成LiNixMn2-xO4(x≤0.10)及电化学性能研究[J]. 电池工业, 2018, 22(2):62-69. doi: 10.3969/j.issn.1008-7923.2018.02.002YU Yue, BAI Hongli, LIU Xiaofang, et al. Study on the synthesis of LiNixMn2-xO4 (x≤0.10) and electrochemical properties via a liquid flameless combustion method[J]. Chinese Battery Industry,2018,22(2):62-69(in Chinese). doi: 10.3969/j.issn.1008-7923.2018.02.002 [21] 朱金玉, 刘清, 向明武, 等. Ni-Cu双掺LiMn2O4正极材料的制备及电化学性能研究[J]. 现代化工, 2015, 43(1):109-115.ZHU Jinyu, LIU Qing, XIANG Mingwu, et al. Preparation of Ni-Cu co-doped LiMn2O4 cathode material and study on its electrochemical properties[J]. Modern Chemical Industry,2015,43(1):109-115(in Chinese). [22] 刘先平, 王桂赟, 宁利娜, 等. 液相燃烧法合成CuCrO2及其与WO3复合后的光催化产氢性能[J]. 硅酸盐学报, 2015, 43(1):109-115.LIU Xianping, WANG Guiyun, NING Lina, et al. Synthesis of CuCrO2 by solution combustion reaction method and photocatalytic hydrogen property of CuCrO2 composite with WO3[J]. Journal of the Chinese Ceramic Society,2015,43(1):109-115(in Chinese). [23] WANG F X, XIAO S Y, SHI Y, et al. Spinel LiNixMn2-xO4 as cathode material for aqueous rechargeable lithium batteries[J]. Electrochimica Acta,2013,93:301-306. [24] CAI Y J, HANG Y D, WANG X C, et al. Facile synthesis of LiMn2O4 octahedral nanoparticles as cathode materials for high capacity lithium ion batteries with long cycle life[J]. Journal of Power Sources,2015,278:574-581. [25] ARIYOSHI K, IWATA E, KUNIYOSHI M, et al. Lithium aluminum manganese oxide having spinel-framework structure for long-life lithium-ion batteries[J]. Electrochemical and Solid-State Letters,2006,9(12):A557-A560. [26] WANG S M, XIANG M W, LU Y, et al. Facile solid-state combustion synthesis of Al-Ni dual-doped LiMn2O4 cathode materials[J]. Journal of Materials Science: Materials in Electronics,2020,31(8):6036-6044. [27] HUANG S S, WU H, CHEN P H, et al. Facile pH-mediated synthesis of morphology-tunable MnCO3 and their transformation to truncated octahedral spinel LiMn2O4 cathode materials for superior lithium storage[J]. Journal of Materials Chemistry A,2015,3(7):3633-3640. [28] JIANG R Y, CUI C Y, MA H Y, et al. Study on the enhanced electrochemical performance of LiMn2O4 cathode material at 55℃ by the nano Ag-coating[J]. Journal of Electroanalytical Chemistry,2015,744:69-76. [29] CHOU S L, WANG J Z, LIU H K, et al. Rapid synthesis of Li4Ti5O12 microspheres as anode materials and its binder effect for lithium-ion battery[J]. The Journal of Physical Chemistry C,2011,115(32):16220-16227. [30] DUAN Y Z, GUO J M, XIANG M W, et al. Single crystalline polyhedral LiNixMn2-xO4 as high-performance cathodes for ultralong cycling lithium-ion batteries[J]. Solid State Ionics,2018,326:100-109. [31] YU Y, XIANG M W, GUO J M, et al. Enhancing high-rate and elevated-temperature properties of Ni-Mg co-doped LiMn2O4 cathodes for Li-ion batteries[J]. Journal of Colloid and Interface Science,2019,555:64-71. -





 下载:
下载: