Study on preparation of two-dimensional Ti3C2Tx nanomaterials modified by sulfonic acid groups and the adsorption performance of lead(II) ion
-
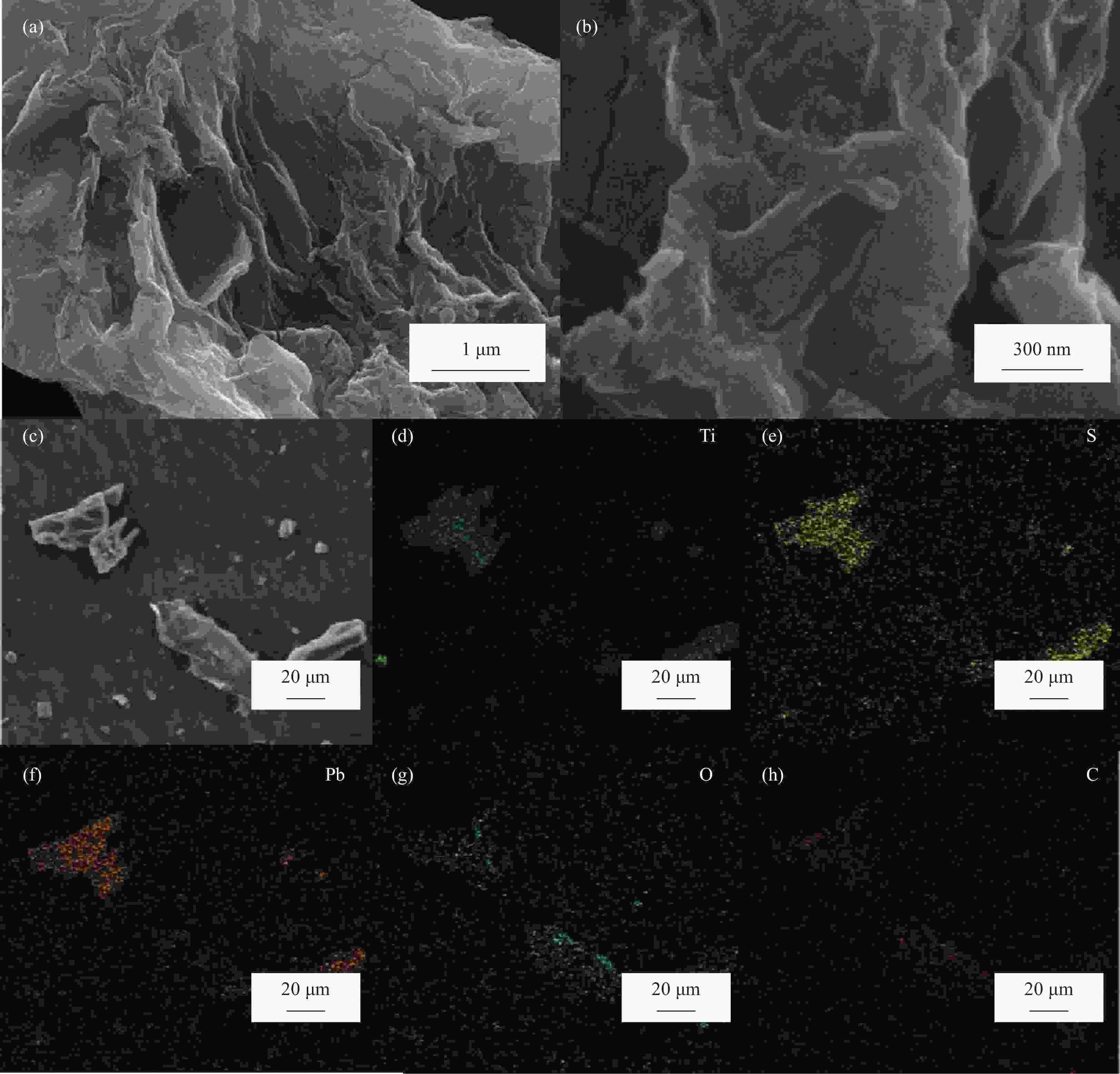
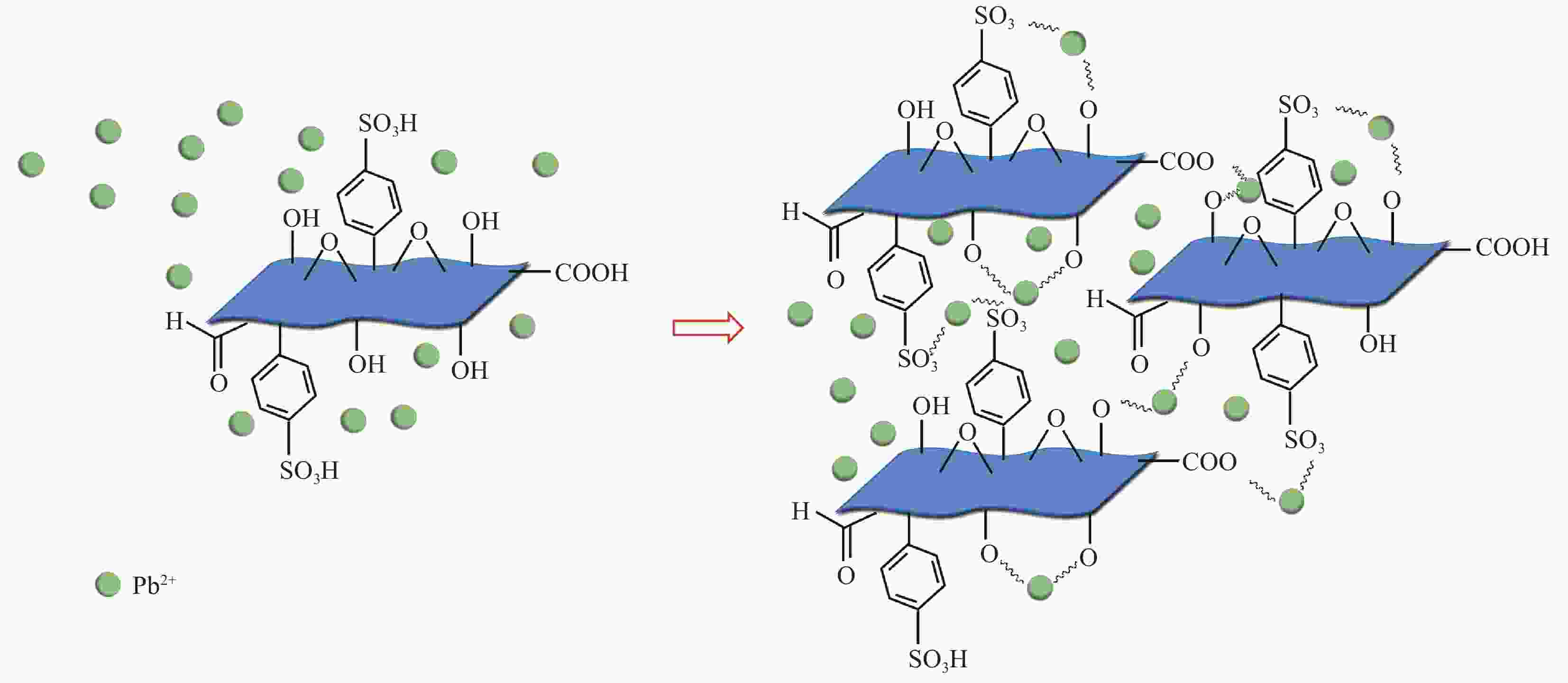
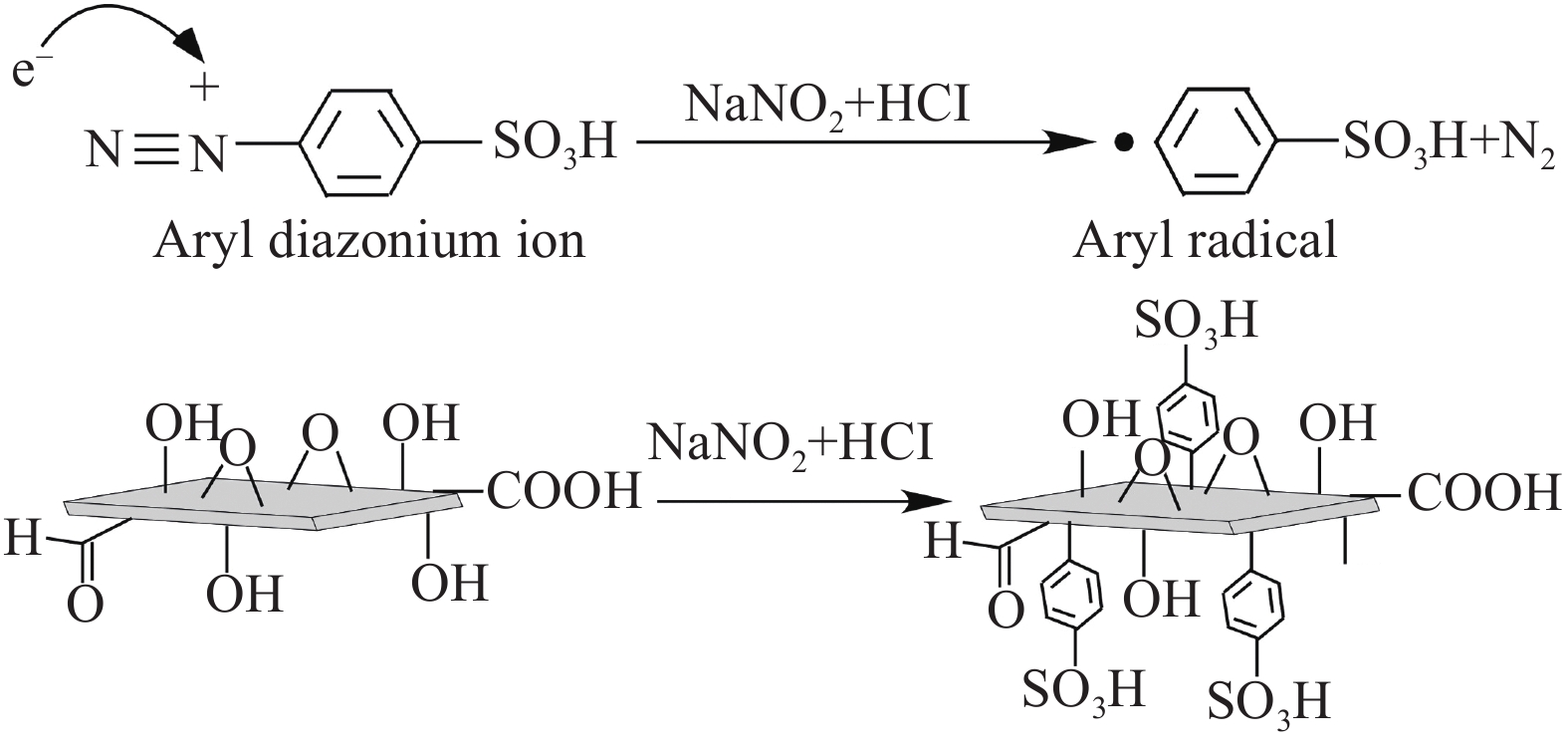
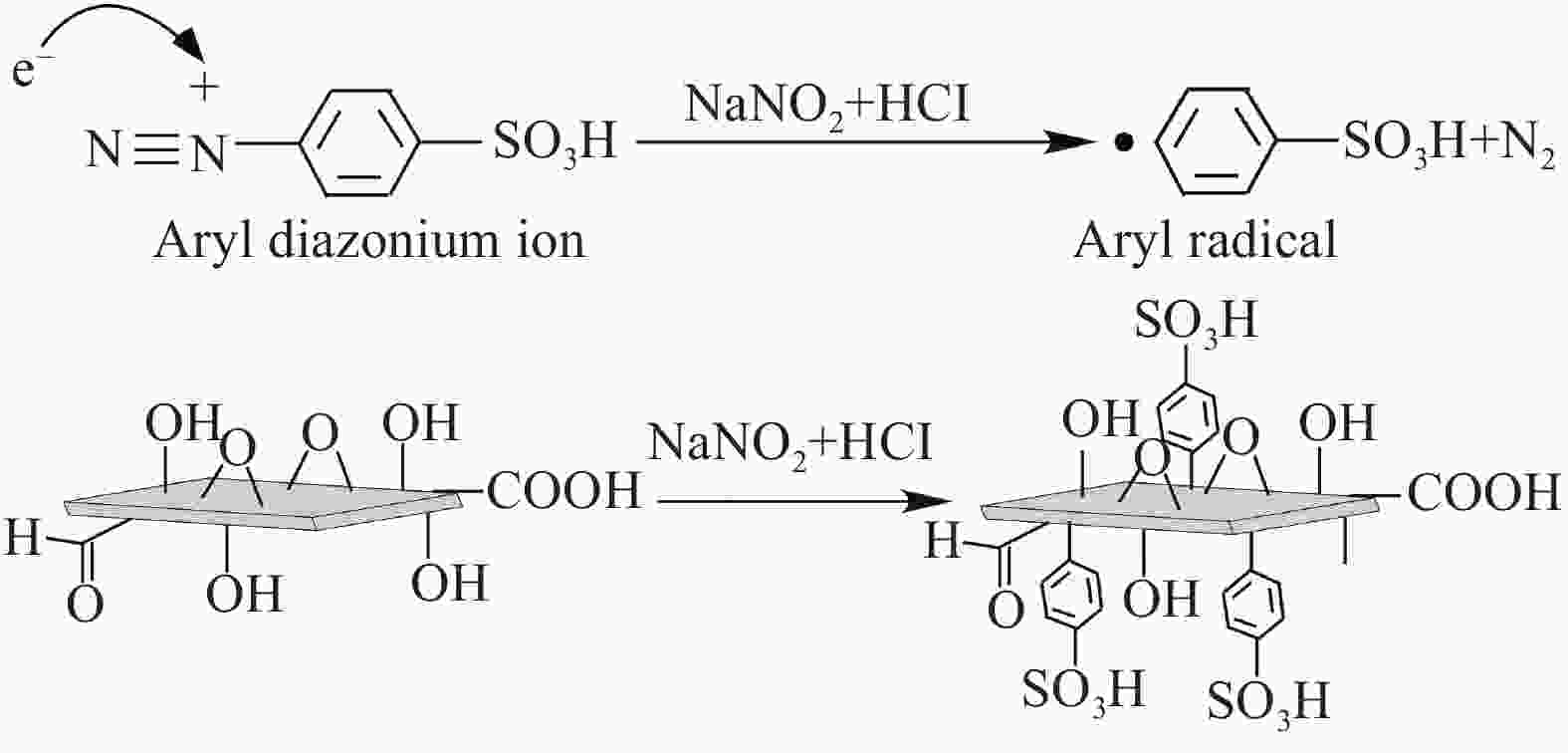
摘要: 对二维Ti3C2Tx材料进行了磺酸基团接枝改性(Ti3C2Tx—SO3H),表征了改性前后微观结构的变化,研究了对重金属Pb2+的吸附行为与机制。XRD、FTIR及EDS表明磺酸基团在Ti3C2Tx表面成功接枝,而SEM则发现Ti3C2Tx−SO3H呈现较Ti3C2Tx更轻薄的层状结构。改性后,Ti3C2Tx—SO3H对重金属Pb2+20 min内达到吸附平衡,最大吸附量达到733.6 mg·g−1,较Ti3C2Tx吸附量提升了23%,且吸附量随着pH(2~6)的增加而逐渐增大,在Mg2+、Ca2+、Co2+、Zn2+等共存离子的干扰下,仍保持较高的吸附水平。机制分析表明,改性前后吸附过程均符合准二级动力学模型和Langmuir吸附等温线拟合模型,以单分子层化学吸附为主,但由于磺酸基团提供了更多的吸附饱和活性位点,并提高了Ti3C2Tx在水溶液中的分散性,使改性后对Pb2+吸附性能更优异。
-
关键词:
- Ti3C2Tx /
- Ti3C2Tx—SO3H /
- 接枝改性 /
- Pb2+ /
- 吸附
Abstract: The two-dimensional Ti3C2Tx was modified by sulfonic acid group grafting (abbreviated as Ti3C2Tx—SO3H), and its microstructure before and after the modification was characterized. The adsorption behavior and mechanism of heavy metal Pb2+ by the Ti3C2Tx—SO3H were also investigated. XRD, FTIR and EDS analyses indicate that sulfonic acid group is successfully grafted on the surface of the Ti3C2Tx, while SEM shows that the Ti3C2Tx—SO3H has a lighter and thinner layered structure than the Ti3C2Tx. Pb2+ adsorption by the Ti3C2Tx—SO3H reaches equilibrium within 20 minutes. The maximum adsorption capacity of the Ti3C2Tx—SO3H is 733.6 mg·g−1, which is 23% higher than the Ti3C2Tx. And Pb2+ adsorption capacity by the Ti3C2Tx—SO3H gradually increases with the increase of pH (2-6). Under the interference of coexisting ions such as Mg2+, Ca2+, Co2+ and Zn2+, the Ti3C2Tx-SO3H still maintains a high level of Pb2+ adsorption. Pb2+ adsorption processes by both the Ti3C2Tx and the Ti3C2Tx—SO3H fit well the pseudo second kinetic model and Langmuir isotherm model, suggesting that the adsorption processes are monolayer chemisorption. The Ti3C2Tx—SO3H shows more excellent adsorption performance towards Pb2+ after adsorption mainly due to the more active sites provided by sulfonic acid group and the enhanced dispersion in aqueous solution.-
Key words:
- Ti3C2Tx /
- Ti3C2Tx—SO3H /
- graft modification /
- Pb2+ /
- adsorption
-
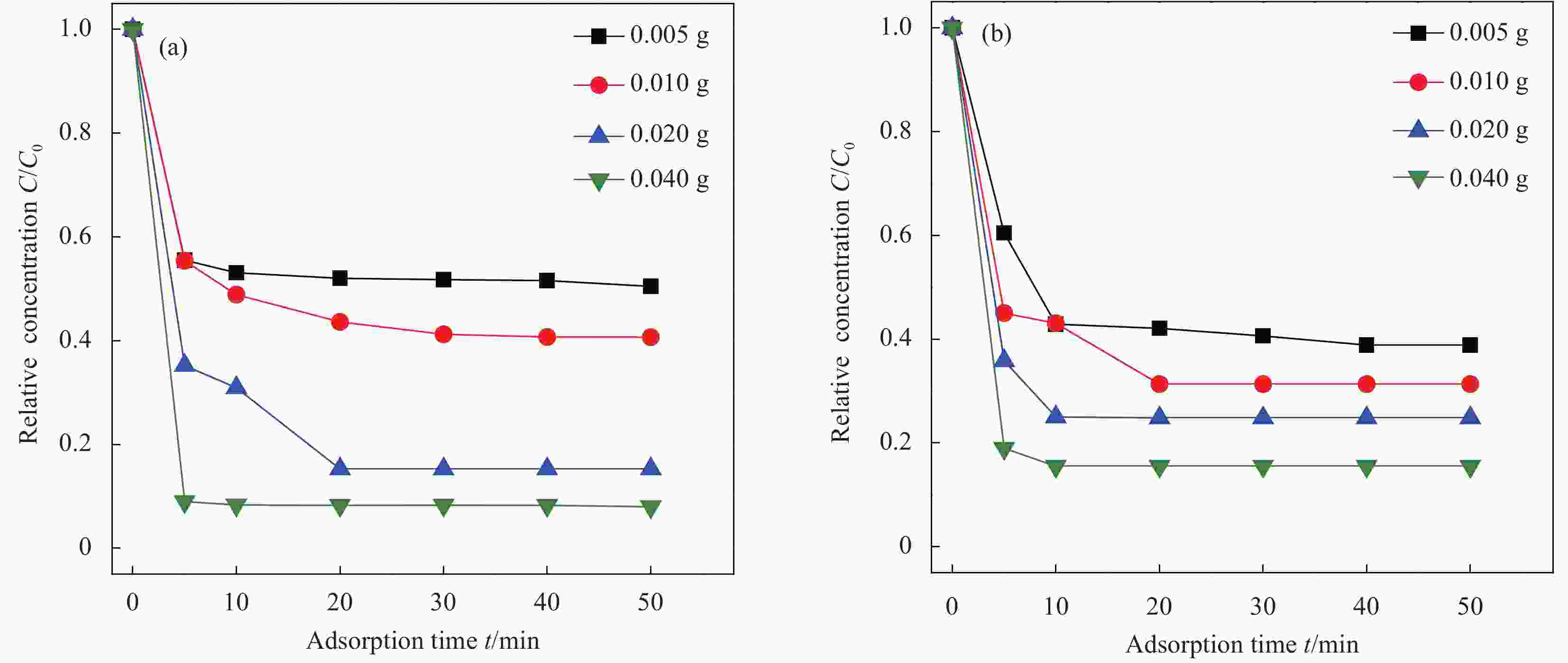
图 11 Ti3C2Tx ((a1), (a2), (a3)) 和Ti3C2Tx—SO3H ((b1), (b2), (b3))吸附Pb2+的动力学模型拟合曲线((a1,b1) 准一级吸附动力学模型;(a2,b2) 准二级吸附动力学模型;(a3, b3) 颗粒内扩散模型)
Figure 11. Kinetic equation fitting curves of Pb2+ adsorbed by Ti3C2Tx ((a1), (a2), (a3)) and Ti3C2Tx—SO3H ((b1), (b2), (b3)) ((a1, b1) Pseudo-first-order adsorption kinetic model; (a2, b2) Pseudo-second-order adsorption kinetic model; (a3, b3) Intra-particle diffusion model)
表 1 不同吸附剂对Pb2+的吸附量对比表
Table 1. Comparison table of Pb2+ adsorption capacity of different adsorbents
Adsorbents qmax/(mg·g−1) pH T/℃ Ref. Amino-modified attapulgite 49.0 6.0 25 [26] CNFs/GO/Fe3O4 composite materials 126.5 6.0 25 [27] Saponified muskmelon peel 167.9 4-6.4 25 [28] Seaweed laminaria japonica 279.5 3-4.8 25 [29] Silanized red mud 361.0 6 25 [30] GO-LDH composite materials 387.7 5.2 25 [31] GO 757.6 3.0 25 [32] Ti3C2Tx 38.5 6.0 40 [16] Ti3C2Tx 594.3 6.0 25 This work Ti3C2Tx—SO3H 733.6 5.9 25 This work Notes: qmax—maximum adsorption capacity; CNFs—Cellulose nanofibrills; GO—Graphene oxide; GO-LDH—Graphene oxide-double layer magnesium & aluminum hydroxide. 表 2 25℃下二维Ti3C2Tx和Ti3C2Tx—SO3H吸附Pb2+的吸附等温式参数表
Table 2. Ti3C2Tx and Ti3C2Tx—SO3H adsorption isotherm parameters of Pb2+ at 25℃
Sample Dosage/g Langmuir Freundlich qm/(mg·g−1) KL/(L·mg−1) R2 n kF/(mg1-(1/n) ·L1/n·g−1) R2 Ti3C2Tx 0.02 793.65 0.0001 0.9604 0.0039 1.2616 0.9817 0.04 300.48 0.0229 0.9338 0.0001 1.0442 0.9694 Ti3C2Tx—SO3H 0.005 199.01 0.0073 0.9366 − − − 0.01 811.10 0.0003 0.9309 − − − Notes: qm—Maximum saturation adsorption capacity; R—Correlation coefficient; KL—Langmuir adsorption equilibrium constant; KF, n—Freundlich adsorption equilibrium constant, denotes adsorption capacity and density in adsorption, respectively. -
[1] 任春溶. 磁性纳米复合材料的制备及其对重金属离子的吸附性能研究[D]. 杭州: 浙江大学, 2017.REN Chunrong. Preparation of magnetic nanocomposites for adsorption of heavy metal ions[D]. Hangzhou: Zhe-jiang University, 2017(in Chinese). [2] LIANG Qianqian, YU Liuhua, JIANG Wei, et al. One-pot synthesis of magnetic graphitic carbon nitride photocatalyst with synergistic catalytic performance under visible-light irradiation[J]. Journal of Photochemistry and Photobiology A: Chemistry,2017,335:165-173. doi: 10.1016/j.jphotochem.2016.11.012 [3] 洪亚军, 冯承莲, 徐祖信, 等. 重金属对水生生物的毒性效应机制研究进展[J]. 环境工程, 2019, 37(11):1-9.HONG Yajun, FENG Chenglian, XU Zuxin, et al. Advances on ecotoxicity effects of heavy metals to aquatic organisms and the mechanisms[J]. Environmental Engineering,2019,37(11):1-9(in Chinese). [4] SONG Yueqin, ZHOU Xiaolong, WANG Jin an. Adsorption performance of activated carbon for methane with low concentration at atmospheric pressure[J]. Energy Sources Part A Recovery Utilization and Environmental Effects,2021,43(11):1337-1347. doi: 10.1080/15567036.2019.1636903 [5] 武雪梅. 天然矿物类吸附剂的复合改性及脱氮除磷性能研究[D]. 武汉: 华中科技大学, 2019.WU Xuemei. Study on the compound modification of natural mineral adsorbents to remove nitrogen and phosphorus[D]. Wuhan: Huazhong University of Science & Technology, 2019(in Chinese). [6] 林驰浩, 徐劼, 王嘉俊, 等. 生物质材料在重金属废水处理中的应用及其研究进展[J]. 广州化工, 2020, 48(5):24-26, 104. doi: 10.3969/j.issn.1001-9677.2020.05.014LIN Chihao, XU Jie, WANG Jiajun, et al. Application and research progress on biomass materials in heavy metal wastewater treatment[J]. Guangzhou Chemical Industry,2020,48(5):24-26, 104(in Chinese). doi: 10.3969/j.issn.1001-9677.2020.05.014 [7] ZHOU Dawei. Multifunctional MXene nanosheets as host materials with superior adsorption ability for enhanced electrochemical performance[J]. Ionics,2021,27:1291-1296. doi: 10.1007/s11581-020-03863-4 [8] ZHANG Shan, TIAN Lidong, CHEN Xiaohu, et al. Ultralight graphene/carbon nanofibers/carbon nanotubes aerogels with thermal insulating and hot-oil adsorption performance[J]. Journal of Materials Science,2021,56(11):7409-7419. [9] ABGHOUI Y, SIGTRYGGSSON S B, EGILL S. Biomimetic nitrogen fixation catalyzed by transition metal sulfide surfaces in an electrolytic cell[J]. ChemSusChem,2019,12(18):4265-4273. doi: 10.1002/cssc.201901429 [10] SUN Yingzhu, CHEN Ming, LIU Hao, et al. Adsorptive removal of dye and antibiotic from water with functionalized zirconium-based metal organic framework and graphene oxide composite nanomaterial UiO-66-(OH)2/GO[J]. Applied Surface Science,2020,525:146614. doi: 10.1016/j.apsusc.2020.146614 [11] HE Peng, CAO Maosheng, CAI Yongzhu, et al. Self-assembling flexible 2D carbide MXene film with tunable integrated electron migration and group relaxation toward energy storage and green EMI shielding[J]. Carbon,2020,157:80-89. doi: 10.1016/j.carbon.2019.10.009 [12] YASAEI P, TU Qing, XU Yaobin, et al. Mapping hot spots at heterogeneities of few-layer Ti3C2 MXene Sheets[J]. ACS Nano,2019,13(3):3301-3309. doi: 10.1021/acsnano.8b09103 [13] 周晓伟. Ti3C2Tx的制备表征及其SERS应用研究[D]. 天津: 天津大学, 2018.ZHOU Xiaowei. The synthesis, characterization of Ti3C2Tx and applying on SERS study[D]. Tianjin: Tianjin University, 2018(in Chinese). [14] ZHANG Yujuan, LAN Jianhui, WANG Lin et al. Adsorption of uranyl species on hydroxylated titanium carbide nanosheet: A first-principles study[J]. Journal of Hazardous Materials,2016,308(308):402-410. [15] FARD A K, MCKAY G, CHAMOUN R, et al. Barium removal from synthetic natural and produced water using MXene as two dimensional (2D) nanosheet adsorbent[J]. Chemical Engineering Journal,2017,317:331-342. doi: 10.1016/j.cej.2017.02.090 [16] JUN B M, HER N G, PARK C M, et al. Effective removal of Pb (II) from synthetic wastewater using Ti3C2Tx MXene[J]. Environmental Science: Water Research & Technology,2020,6(1):173-180. [17] 田程程. 新型多功能纳米复合材料的合成及其催化性能研究[D]. 上海: 华东理工大学, 2014.TIAN Chengcheng. Synthesis and catalytic performance of novel multifunctional nanocomposites[D]. Shanghai: East China University of Science and Technology, 2014(in Chinese). [18] ZHANG Pengcheng, WANG Lin, HUANG Zhiwei, et al. Aryl diazonium-assisted amidoximation of MXene for boosting water stability and uranyl sequestration via electrochemical sorption[J]. ACS Applied Materials & Interfaces,2020,12:15579-15587. [19] SEHYEONG L, HYUNSU P, JIN H K, et al. Polyelectrolyte-grafted Ti3C2-MXenes stable in extreme salinity aquatic conditions for remediation of contaminated subsurface environments[J]. RSC Advances,2020,10:25966-25978. doi: 10.1039/D0RA04348F [20] LI Yahui, DENG Yanan, ZHANG Jianfeng, et al. Synthesis of restacking-free wrinkled Ti3C2Tx monolayers by sulfonic acid group grafting and N-doped carbon decoration for enhanced supercapacitor performance[J]. Journal of Alloys and Compounds,2020,842:155985. doi: 10.1016/j.jallcom.2020.155985 [21] LI Yahui, DENG Yanan, ZHANG Jianfeng, et al. Tunable energy storage capacity of two-dimensional Ti3C2Tx modified by a facile two-step pillaring strategy for high performance supercapacitor electrodes[J]. Nanoscale,2019,11(45):21981-21989. doi: 10.1039/C9NR07259D [22] OBAID S A. Langmuir, Freundlich and Tamkin adsorption isotherms and kinetics for the removal aartichoke tournefortii straw from agricultural waste[J]. Journal of Physics Conference Series,2020,1664:012011. doi: 10.1088/1742-6596/1664/1/012011 [23] MARCU C, VARODI C, BALLA A. Adsorption kinetics of chromium (VI) from aqueous solution using an anion exchange resin[J]. Analytical Letters,2020,54:140-149. [24] SI Y C, SAMULSKI E T. Synthesis of water soluble graphene[J]. Nano Letters,2008,8:1679-1682. doi: 10.1021/nl080604h [25] SUGANUMA S, NAKAJIMA K, KITANO M et al. SO3H-bearing mesoporous carbon with highly selective catalysis[J]. Microporous and Mesoporous Materials,2011,143(2-3):443-450. doi: 10.1016/j.micromeso.2011.03.028 [26] XU Lei, LIU Yani, WANG Jingang, et al. Selective adsorption of Pb2+ and Cu2+ on amino-modified attapulgite: kinetic, thermal dynamic and DFT studies[J]. Journal of Hazardous Materials,2021,404:124140. doi: 10.1016/j.jhazmat.2020.124140 [27] HOSSEINI M, DIZAJI H Z, TAGHAVI M, et al. Preparation of ultra-lightweight and surface-tailored cellulose nanofibril composite cryogels derived from date palm waste as powerful and low-cost heavy metals adsorbent to treat aqueous medium[J]. Industrial Crops and Products,2020,154:112696. doi: 10.1016/j.indcrop.2020.112696 [28] HUANG Kai, ZHU Hongmin. Removal of Pb2+ from aqueous solution by adsorption on chemically modified muskmelon peel[J]. Environmental Science and Pollution Research International,2013,20(7):4424-4434. doi: 10.1007/s11356-012-1361-7 [29] GHIMIRE K N, INOUE K, OHTO K, et al. Adsorption study of metal ions onto crosslinked seaweed Laminaria japonica[J]. Bioresource Technology,2008,99(1):32-37. doi: 10.1016/j.biortech.2006.11.057 [30] 刘江龙, 郭焱, 何小山, 等. 硅烷化赤泥的制备及其对水中铅离子吸附性能分析[J]. 环境工程, 2019, 37(11):36-44.LIU Jianglong, GUO Yan, HE Xiaoshan, et al. Preparation of silanized red mud and its adsorption properties for lead ions in water[J]. Environmental Engineering,2019,37(11):36-44(in Chinese). [31] 周博秋, 李慧强, 廖维, 等. GO-LDH复合材料对水中铅离子的吸附性能[J]. 中国给水排水, 2019, 35(17):105-111.ZHOU Boqiu, LI Huiqiang, LIAO Wei, et al. Adsorption properties of GO-LDH composite materials to lead ion in water[J]. China Water & Wastewater,2019,35(17):105-111(in Chinese). [32] 梁程. 还原条件下氧化石墨烯对铅离子吸附/解吸附性能的研究[D]. 西安: 西安建筑科技大学, 2016.LIANG Cheng. A study on oxide graphene’s adsorption and desorption property of lead in the reductive condition[D]. Xi’an: Xi’an University of Architecture and Technology, 2016(in Chinese). -





 下载:
下载: