MoS2 catalyzed peroxymonosulfate activation for organic pollutants degradation: A review
-
摘要: 随着城镇化进程加快,大量含难降解有机污染物的工业废水和生活污水因不合理处置而进入水体,对水环境质量造成严重威胁。过渡金属离子催化活化单过硫酸盐(PMS)产生活性氧去除水中难降解有机物的催化体系的研究已有大量文献报道,但存在金属离子二次污染和催化剂难以回收等问题。MoS2作为优异的二维半导体材料,在储能和催化领域颇具优势并实现产业化生产。在水处理领域,研究发现MoS2作为非均相金属催化剂能够有效活化PMS去除水中难降解有机物。本文主要综述了MoS2作为催化剂、金属离子助催化剂或复合型共催化剂活化PMS体系降解水中有机污染物的研究进展,归纳并比较上述催化体系对污染物的降解效能,对催化反应机制进行探讨分析,并针对目前存在的问题提出相关研究展望。Abstract: With the acceleration of urbanization, the massive discharge of industrial wastewater and domestic sewage containing refractory organic pollutants has entered into waterbodies due to unreasonable disposal, which poses a serious threat to the water environment quality. Transition-metal ions mediated peroxymonosulfate (PMS) activation has been extensively studied as their ability to produce various active oxygen species for contaminants removal in water. However, the accompanied problems of secondary pollution and difficulty in catalyst recovery has limited their practical application. MoS2, an excellent two-dimensional semiconductor material of industrial production, has been widely used in energy conversion and catalysis area. In water treatment, MoS2 materials have been reported as an efficient heterogeneous catalyst for PMS activation to degrade refractory organic pollutants. This review summarized recent studies concerning MoS2-catalyzed PMS activation for organic pollutants removal in aqueous solution. The catalysis efficiencies of PMS activation mediated by MoS2, MoS2/metal ions and MoS2 supported materials were comparatively described, the relevant mechanisms were discussed and the development prospect of the current research were expected briefly.
-
Key words:
- MoS2 /
- peroxymonosulfate /
- active oxygen /
- degradation /
- organic pollutants
-
表 1 不同MoS2/Fe(Ⅱ)/PMS催化体系对有机污染物降解性能及机制比较
Table 1. Comparison of performance and mechanism of different MoS2/Fe(Ⅱ)/PMS systems
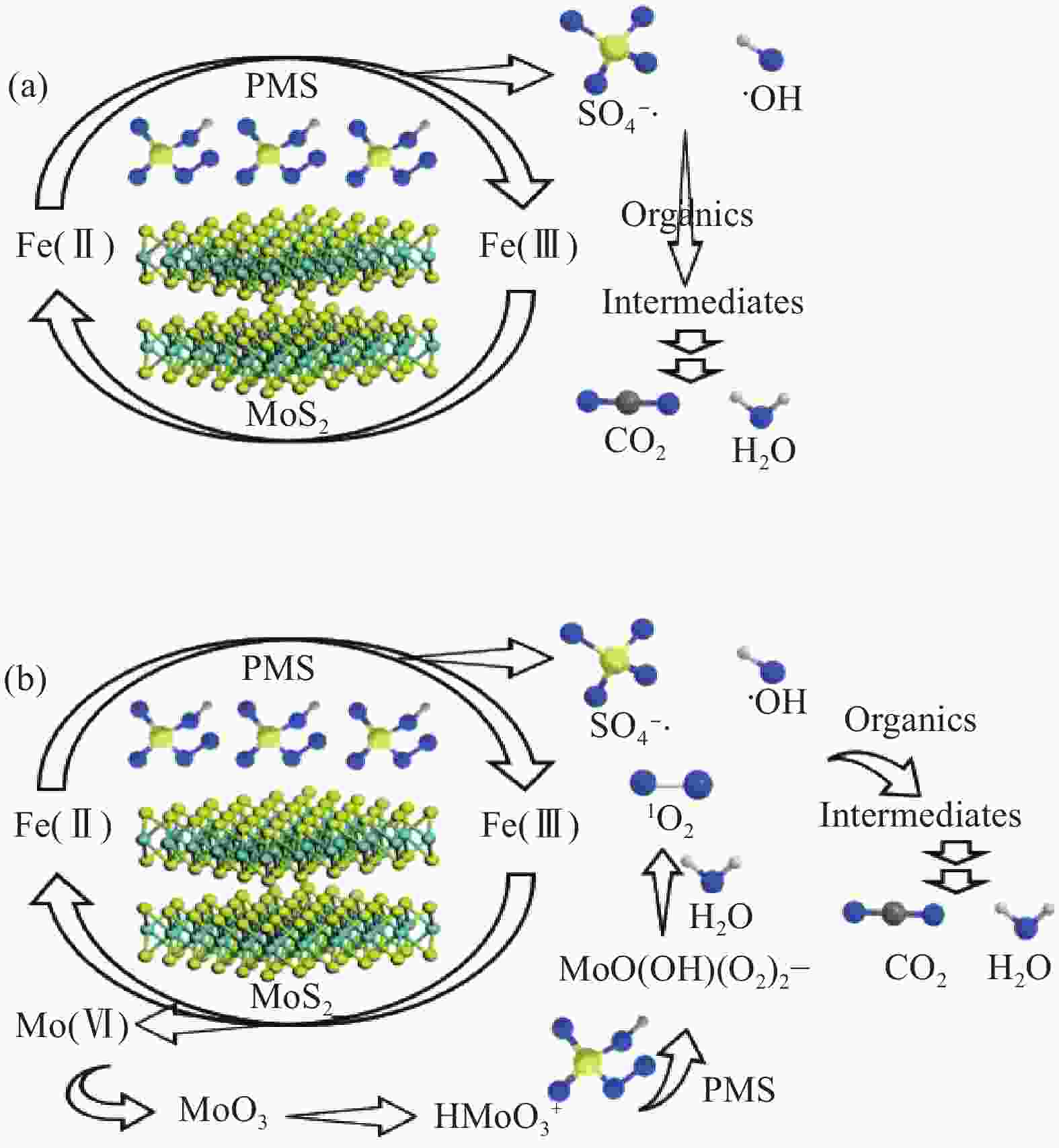
Number ① ② ③ ④ Reference [47] [48] [49] [50] Reaction condition [ACT]=20 mg/L, [RhB]=20 mg/L, [TCP]=19.7 mg/L, [SMX]=6.3 mg/L, [Fe(Ⅱ)]=1 mg/L, [MoS2]=0.1 g/L, [PMS]=1 mmol/L pH=3 [Fe(Ⅱ)]=3 mg/L, [MoS2]=0.3 g/L, [PMS]=1 mmol/L pH=3 [Fe(Ⅱ)]=2.26 mg/L, [MoS2]=1 g/L, [PMS]=0.5 mmol/L pH=3 [Fe(Ⅱ)]=3.92 mg/L, [MoS2]=0.3 mg/L, [PMS]=0.075 mmol/L pH=3 Pollutant ACT RhB TCP SMX Main active oxygen 1O2 SO4−·, ·OH SO4−·, ·OH SO4−·, ·OH Efficiency 60 min, 100% 60 min, 94.7% 30 min, >95% 6 min, 88.5% TOC 29.8% 59.8% >25% 33.9% Ion leaching Mo: 60 min, 2.88 mg/L — Mo: 30 min, >8 mg/L — Recycle 6 times, >90% 8 times, >95% 5 times, 93% 6 times, ~90% Mechanism (1) Fe(Ⅱ)+HSO5− → Fe(Ⅲ)+SO4−·+OH−
(2) Mo(Ⅳ)+ 2Fe(Ⅲ) → 2Fe(Ⅱ)+Mo(Ⅵ)
(3) Mo(Ⅵ) → … → MoO3
(4) MoO3+H+→HMoO3+ +2HSO5− → MoO(OH)(O2)2−+2SO3+H2O
(5) MoO(OH)(O2)2−+ H2O→MoO42−+1O2+H+
(6) 1O2+ACT→H2O+CO2+others(1) Fe(Ⅱ)+HSO5− → Fe(Ⅲ)+SO4−·+OH−
(2) Fe(Ⅱ)+HSO5− → Fe(Ⅲ)+SO4−+·OH
(3) Mo(Ⅳ)+2Fe(Ⅲ) → 2Fe(Ⅱ)+Mo(Ⅵ)
(4) Fe(Ⅲ)+HSO5− → Fe(Ⅱ)+SO5−·+H+
(5) Mo(Ⅵ)+2HSO5−→Mo(Ⅳ)+2SO5−·+ H+
(6) ·OH/SO4−·+RhB/TCP/SMX → H2O+CO2+othersNotes: TOC—Total organic carbon; ACT—Acetaminophen; RhB—Rhodamine B; TCP—2,4,6-Trichlorophenol; SMX—Sulfamethoxazole. 表 2 非均相MoS2/PMS降解有机污染物催化体系汇总
Table 2. Summary of heterogeneous MoS2/PMS system for degradation of organic pollutants
Category System Pollutant Reaction condition Efficiency Ref. Single MoS2 1T-MoS2/PMS BPA [BPA]=2 mg/L,
[MoS2]=15 mg/L,
[PMS]=50 mg/L
pH=4, T=25℃10 min, 91% [36] 2H-MoS2/PMS CBZ [CBZ]=5 mg/L,
[MoS2]=0.2 g/L,
[PMS]=0.2 mmol/L
pH=3~9, T=30℃40 min, > 95% [42] Co-catalytic
MoS2/PMSMoS2/Fe(Ⅱ)/PMS ACT [ACT]=20 mg/L,
[Fe(Ⅱ)]=3 mg/L,
[MoS2]=0.3 g/L,
[PMS]=1 mmol/L
pH=3, T=25℃60 min, 100% [47] MoS2/Fe(Ⅱ)/PMS RhB [RhB]=20 mg/L,
[Fe(Ⅱ)]=1 mg/L,
[MoS2]=0.1 g/L,
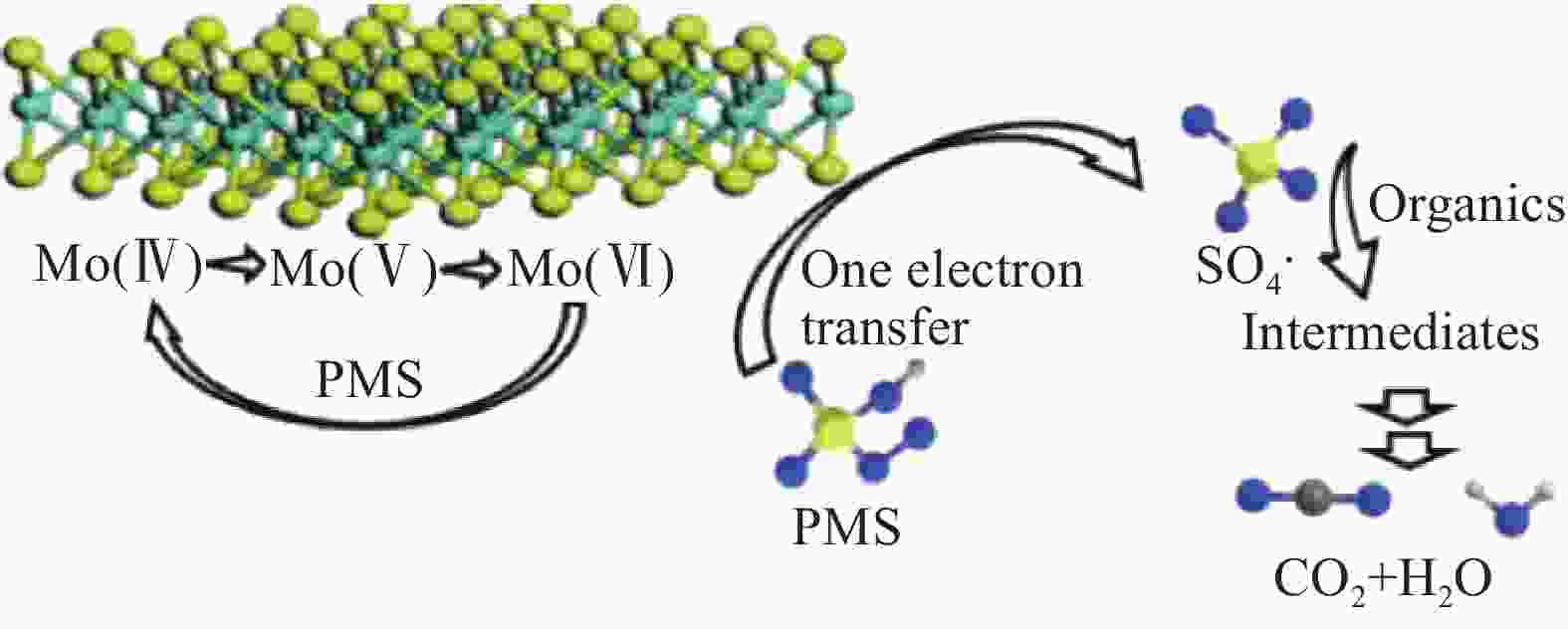
[PMS]=1 mmol/L
pH=3, T=25℃60 min, 100% [48] MoS2/Fe(Ⅱ)/PMS TCP [TCP]=19.7 mg/L,
[Fe(Ⅱ)]=2.26 mg/L,
[MoS2]=1 g/L,
[PMS]=0.5 mmol/L
pH=3, T=25℃60 min, 100% [49] MoS2/Fe(Ⅱ)/PMS SMX [SMX]=6.3 mg/L,
[Fe(Ⅱ)]=3.92 mg/L,
[MoS2]=0.3 g/L,
[PMS]=0.075 mmol/L
pH=3, T=25℃60 min,100% [50] MoS2/Fe(Ⅲ)/PMS RhB [RhB]=20 mg/L,
[Fe(Ⅲ)]=5.6 mg/L,
[MoS2]=0.3 g/L,
[PMS]=1.5 mmol/L
pH=3, T=25℃60 min, 95% [51] MoS2/Co(Ⅱ)/PMS RhB [RhB]=30 μmol/L,
[Co(Ⅱ)]=2 μmol/L,
[MoS2]=0.5 g/L,
[PMS]=0.2 mmol/L pH=5.55 min, 100% [53] Loaded MoS2/PMS SMG/Fe(Ⅱ)/PMS SD [SD]=20 mg/L,
[Fe(Ⅱ)]=0.25 mg/L,
[SMG loaded MoS2]=1 g/L,
[PMS]=150 mg/L, T=25℃30 min, 98.3% [59] MoS2/CuFe2O4/PMS Fluoxetine [Fluoxetine]=20 mg/L,
[MoS2/CuFe2O4]=0.1 g/L,
[PMS]=1 mmol/L
pH=6.9, T=25℃20min, 97.3% [60] Vis/3D urchin-like MoS2/C/PMS LEV [LEV]=70 mg/L,
[3D urchin-like MoS2/C]=0.1 g/L,
[PMS]=0.9 g/L
Vis: 30 mW·cm−2, 420 nm
pH=7, T=25℃80min, 100% [61] Vis/WO3/MoS2/Ag/PMS BPA [BPA]=10 mg/L,
[WO3/MoS2/Ag]=0.8 g/L,
[PMS]=1 g/L
Vis: 2 000 mW·cm−2, 420 nm
pH=9, T=25℃140 min, > 90% [62] Vis/MoS2/Ag/g-C3N4/PMS TC [TC]=20 mg/L,
[MoS2/Ag/g-C3N4]=0.2 g/L,
[PMS]=0.1 mmol/L
Vis: 300 W, 420 nm
pH=5.5, T=20℃50 min, 98.9% [63] Doped MoS2/PMS FexMo1−xS2/PMS PPA [PPA]=10 μmol/L
[FexMo1−xS2]=0.1 g/L,
PMS=1 mmol/L,pH=4.030 min, 90% [65] Vis/Co-MoS2/PMS OFX [OFX]=20 mg/L,
[Co-MoS2]=0.1 g/L,
[PMS]=0.75 g/L
Vis: 300 W, 420 nm pH=9, T=20℃30 min, 91.1% [66] Notes: BPA—Bisphenol A; CBZ—Carbamazepine; SD—Sulfadiazine; LEV—Levofloxacin; TC—Tetracycline; PPA—Propranolol; OFX—Ofloxacian; T—Temperature. -
[1] LIU J L, WONG M H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China[J]. Environment International,2013,59:208-224. doi: 10.1016/j.envint.2013.06.012 [2] 赵金平. 我国持久性有机污染物监测现状、存在问题及对策分析[J]. 环境与发展, 2019, 31(5):55-56.JINPING Z. Current status, existing problems and countermeasures of monitoring persistent organic pollutants in China[J]. Environment and Development,2019,31(5):55-56(in Chinese). [3] GLAZE W H, KANG J W, CHAPIN D H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation[J]. Ozone Science & Engineering,1987,9(4):335-352. [4] DENG Y, ZHAO R. Advanced oxidation processes (AOPs) in wastewater treatment[J]. Current Pollution Reports,2015,1(3):167-176. doi: 10.1007/s40726-015-0015-z [5] PRPIĆ D. Advanced oxidation processes (AOPs) in the treatment of reactive dyes wastewater[D]. Zagreb: Fakultet Kemijskog Inženjerstva i Tehnologije, 2004. [6] CENTI G, PERATHONER S. Advanced oxidation processes in water treatment[M]//DANIEL D, FABRIZIO C. Handbook of advanced methods and processes in oxidation catalysis. Imperial College Press, 2014. [7] KURT A, MERT B K, ÖZENGIN N, et al. Treatment of antibiotics in wastewater using advanced oxidation processes (AOPs)[M]//FAROOQ R, AHMAD Z. Physico-chemical wastewater treatment and resource recovery. IntechOpen, 2017. [8] PLIEGO G, ZAZO J A, GARCIA-MUÑOZ P, et al. Trends in the intensification of the fenton process for wastewater treatment: An overview[J]. Critical Reviews in Environmental Science & Technology,2015,45(24):2611-2692. [9] NIDHEESH P V, GANDHIMATHI R. Trends in electro-Fenton process for water and wastewater treatment: An overview[J]. Desalination,2012,299:1-15. [10] FOTEINIS S, MONTEAGUDO J M, DURÁN A, et al. Environmental sustainability of the solar photo-Fenton process for wastewater treatment and pharmaceuticals mineralization at semi-industrial scale[J]. Science of the Total Environment,2017,612:605-612. [11] WANG Q, TIAN S, NING P. Degradation mechanism of methylene blue in a heterogeneous Fenton-like reaction catalyzed by ferrocene[J]. Industrial & Engineering Chemistry Research,2014,53(2):643-649. [12] ZHOU X, LUO C, LUO M, et al. Understanding the synergetic effect from foreign metals in bimetallic oxides for PMS activation: A common strategy to increase the stoichiometric efficiency of oxidants[J]. Chemical Engineering Journal,2020,381:122587. doi: 10.1016/j.cej.2019.122587 [13] YANG S, HE H, WU D, et al. Degradation of methylene blue by heterogeneous Fenton reaction using titanomagnetite at neutral pH values: Process and affecting factors[J]. Industrial & Engineering Chemistry Research,2009,48(22):9915-9921. [14] GHANBARI F, MORADI M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review[J]. Chemical Engineering Journal,2016,310:41-62. [15] WANG J, WANG S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal,2018,334:1502-1517. doi: 10.1016/j.cej.2017.11.059 [16] LIM J, YANG Y, HOFFMANN M R. Activation of peroxymonosulfate by oxygen vacancies-enriched cobalt-doped black TiO2 nanotubes for the removal of organic pollutants[J]. Environmental Science & Technology,2019,53(12):6972-6980. [17] GAO Y, CHEN Z, ZHU Y, et al. New insights into the generation of singlet oxygen in the metal-free peroxymonosulfate activation process: Important role of electron-deficient carbon atoms[J]. Environmental Science & Technology,2020,54(2):1232-1241. [18] SUN B, MA W, WANG N, et al. Polyaniline: A new metal-free catalyst for peroxymonosulfate activation with highly efficient and durable removal of organic pollutants[J]. Environmental Science and Technology,2019,53(16):9771-9780. [19] YANG S, WANG P, YANG X, et al. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide[J]. Journal of Hazardous Materials,2010,179(1-3):552-558. doi: 10.1016/j.jhazmat.2010.03.039 [20] LIU J, ZHOU J, DING Z, et al. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye[J]. Ultrasonics Sonochemistry,2017,34:953-959. doi: 10.1016/j.ultsonch.2016.08.005 [21] LIU X, ZHANG T, ZHOU Y, et al. Degradation of atenolol by UV/peroxymonosulfate: Kinetics, effect of operational parameters and mechanism[J]. Chemosphere,2013,93(11):2717-2724. doi: 10.1016/j.chemosphere.2013.08.090 [22] ANIPSITAKIS G P, DIONYSIOU D D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology,2004,38(13):3705-3712. [23] ZHU C, LIU F, LING C, et al. Growth of graphene-supported hollow cobalt sulfide nanocrystals via MOF-templated ligand exchange as surface-bound radical sinks for highly efficient bisphenol A degradation[J]. Applied Catalysis B: Environmental,2019,242:238-248. doi: 10.1016/j.apcatb.2018.09.088 [24] WANG G, NIE X, JI X, et al. Enhanced heterogeneous activation of peroxymonosulfate by Co and N codoped porous carbon for degradation of organic pollutants: The synergism between Co and N[J]. Environmental Science: Nano,2019,6(2):399-410. [25] FAN J, GU L, WU D, et al. Mackinawite (FeS) activation of persulfate for the degradation of p-chloroaniline: Surface reaction mechanism and sulfur-mediated cycling of iron species[J]. Chemical Engineering Journal,2017,333:657-664. [26] SAPUTRA E, MUHAMMAD S, SUN H, et al. A comparative study of spinel structured Mn3O4, Co3O4 and Fe3O4 nanoparticles in catalytic oxidation of phenolic contaminants in aqueous solutions[J]. Journal of Colloid & Interface Science,2013,407(10):467-473. [27] SHI P, SU R, ZHU S, et al. Supported cobalt oxide on graphene oxide: Highly efficient catalysts for the removal of Orange Ⅱ from water[J]. Journal of Hazardous Materials,2012,229-230:331-339. [28] ZHOU R, ZHAO J, SHEN N, et al. Efficient degradation of 2,4-dichlorophenol in aqueous solution by peroxymonosulfate activated with magnetic spinel FeCo2O4 nanoparticles[J]. Chemosphere,2018,197(1):670-679. [29] LOPEZ-SANCHEZ O, LEMBKE D, KAYCI M, et al. Ultrasensitive photodetectors based on monolayer MoS2[J]. Nature Nanotechnology,2013,8(7):497-501. doi: 10.1038/nnano.2013.100 [30] JOENSEN P, FRINDT R F, MORRISON S R. Single-layer MoS2[J]. Materials Research Bulletin,1986,21(4):457-461. doi: 10.1016/0025-5408(86)90011-5 [31] HELVEG, LAURITSEN, LAEGSGAARD, et al. Atomic-scale structure of single-layer MoS2 nanoclusters[J]. Physical Review Letters,2000,84(5):951-954. [32] YIN Z, LI H, LI H, et al. Single-layer MoS2 phototransistors[J]. ACS Nano,2012,6(1):74-80. doi: 10.1021/nn2024557 [33] LATE D, HUANG Y, LIU B, et al. Sensing behavior of atomically thin-layered MoS2 transistors[J]. ACS Nano,2013,7(6):4879-91. doi: 10.1021/nn400026u [34] KUMAR R, GOEL N, KUMAR M. UV-activated MoS2 based fast and reversible NO2 sensor at room temperature[J]. ACS Sensors,2017,2(11):1744-1752. [35] AMIINU I S, PU Z, LIU X, et al. Multifunctional Mo-N/C@MoS2 electrocatalysts for HER, OER, ORR, and Zn-air batteries[J]. Advanced Functional Materials,2017,27(44):1702300. [36] CHEN Y, ZHANG G, LIU H, et al. Confining free radicals in close vicinity to contaminants enables ultrafast Fenton-like processes in the interspacing of MoS2 membranes[J]. Angewandte Chemie International Edition,2019,58(24):8134-8138. [37] WANG Z, MI B. Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets[J]. Environmental Science & Technology,2017,51(15):8229-8244. [38] KUC A, HEINE T. The electronic structure calculations of two-dimensional transition-metal dichalcogenides in the presence of external electric and magnetic fields[J]. Chemical Society Reviews,2015,44(9):2603-2614. doi: 10.1039/C4CS00276H [39] CHEN Y, ZHANG G, JI Q, et al. Triggering of low-valence molybdenum in multiphasic MoS2 for effective reactive oxygen species (ROS) output in catalytic Fenton-like reactions[J]. ACS Applied Materials & Interfaces,2019,11(30):26781-26788. [40] DENG M, KWAC K, LI M, et al. Stability, molecular sieving, and ion diffusion selectivity of a lamellar membrane from two-dimensional molybdenum disulfide[J]. Nano Letters,2017,17(4):2342-2348. doi: 10.1021/acs.nanolett.6b05238 [41] DING L, WEI Y, WANG Y, et al. A two-dimensional lamellar membrane: MXene nanosheet stacks[J]. Angewandte Chemie,2017,129(7):1851-1855. [42] ZHOU H, LAI L, WAN Y, et al. Molybdenum disulfide (MoS2): A versatile activator of both peroxymonosulfate and persulfate for the degradation of carbamazepine[J]. Chemical Engineering Journal,2020,384:123264. doi: 10.1016/j.cej.2019.123264 [43] GÁBOR L, JÓZSEF K, ZSUZSA B, et al. One-versus two-electron oxidation with peroxomonosulfate ion: Reactions with iron(Ⅱ), vanadium(Ⅳ), halide ions, and photoreaction with cerium(Ⅲ)[J]. Inorganic Chemistry,2009,48(4):1763-1773. doi: 10.1021/ic801569k [44] XING M, XU W, DONG C, et al. Metal sulfides as excellent Co-catalysts for H2O2 decomposition in advanced oxidation processes[J]. Chem,2018,4(6):1359-1372. [45] HE D, CHENG Y, ZENG Y, et al. Synergistic activation of peroxymonosulfate and persulfate by ferrous ion and molybdenum disulfide for pollutant degradation: Theoretical and experimental studies[J]. Chemosphere,2019,240:124979. [46] XIAO X, WANG Y, CUI B, et al. Preparation of MoS2 nanoflowers with rich active sites as an efficient adsorbent for aqueous organic dyes[J]. New Journal of Chemistry,2020,44(11):4558-4567. [47] WU Z, FANG B, WANG Z, et al. MoS2 nanosheets: A designed structure with high active site density for the hydrogen evolution reaction[J]. ACS Catalysis,2013,3(9):2101-2107. [48] ZHANG Y, NIU J, XU J. Fe(Ⅱ)-promoted activation of peroxymonosulfate by molybdenum disulfide for effective degradation of acetaminophen[J]. Chemical Engineering Journal,2020,381:122718. doi: 10.1016/j.cej.2019.122718 [49] SHENG B, YANG F, WANG Y, et al. Pivotal roles of MoS2 in boosting catalytic degradation of aqueous organic pollutants by Fe(Ⅱ)/PMS[J]. Chemical Engineering Journal,2019,375:121985. [50] WANG S, XU W, WU J, et al. Improved sulfamethoxazole degradation by the addition of MoS2 into the Fe2+/peroxymonosulfate process[J]. Separation and Purification Technology,2019,235:116170. [51] LUO H, CHENG Y, ZENG Y, et al. Rapid removal of organic micropollutants by heterogeneous peroxymonosulfate catalysis over a wide pH range: Performance, mechanism and economic analysis[J]. Separation and Purification Technology,2020,248:117023. doi: 10.1016/j.seppur.2020.117023 [52] ANIPSITAKIS G P, DIONYSIOU D D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt[J]. Environmental Science & Technology,2003,37(20):4790-4797. [53] PAN C, FU L, DING Y, et al. Homogeneous catalytic activation of peroxymonosulfate and heterogeneous reductive regeneration of Co2+ by MoS2: The pivotal role of pH[J]. Science of the Total Environment, 2020, 712: 136447. [54] ZDRAŽIL M. Preparation of molybdenum-based catalysts by new slurry impregnation method: Active carbon supported molybdenum sulfide hydrodesulfurization catalyst[J]. Applied Catalysis A: General,1994,115(2):285-293. [55] XING W, ZHANG W, XUE Q, et al. Highly active catalyst of two-dimensional CoS2/graphene nanocomposites for hydrogen evolution reaction[J]. Nanoscale Research Letters,2015,10:488. [56] SONG X C, ZHENG Y F, ZHAO Y. Hydrothermal synthesis and characterization of CNT@MoS2 nanotubes[J]. Materials Letters,2006,60(19):2346-2348. doi: 10.1016/j.matlet.2006.01.002 [57] XU H, ZHANG Y, LI J, et al. Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols[J]. Environmental Pollution,2019,257:113610. [58] WANG S, WANG J. Peroxymonosulfate activation by Co9S8@S and N co-doped biochar for sulfamethoxazole degradation[J]. Chemical Engineering Journal,2020,385:123933. doi: 10.1016/j.cej.2019.123933 [59] ZHU L, JI J, LIU J, et al. Designing 3D-MoS2 sponge as excellent cocatalysts in advanced oxidation processes for pollutant control[J]. Angewandte Chemie International Edition,2020,59(33):13968-13976. [60] BAI R, YAN W, XIAO Y, et al. Acceleration of peroxymonosulfate decomposition by a magnetic MoS2/CuFe2O4 heterogeneous catalyst for rapid degradation of fluoxetine[J]. Chemical Engineering Journal,2020,397:125501. doi: 10.1016/j.cej.2020.125501 [61] ZENG L, LI S, LI X, et al. Visible-light-driven sonophotocatalysis and peroxymonosulfate activation over 3D urchin-like MoS2/C nanoparticles for accelerating levofloxacin elimination: Optimization and kinetic study[J]. Chemical Engineering Journal,2019,378:122039. doi: 10.1016/j.cej.2019.122039 [62] ZENG Y, GUO N, NI X, et al. Degradation of bisphenol a using peroxymonosulfate activated by WO3@MoS2/Ag hollow nanotubes photocatalyst[J]. Chemosphere,2019,227:589-597. [63] JIN C, KANG J, LI Z, et al. Enhanced visible light photocatalytic degradation of tetracycline by MoS2/Ag/g-C3N4 Z-scheme composites with peroxymonosulfate[J]. Applied Surface Science,2020,514:146076. doi: 10.1016/j.apsusc.2020.146076 [64] WANG A, LI J, ZHANG T. Heterogeneous single-atom catalysis[J]. Nature Reviews Chemistry,2018,2:65-81. doi: 10.1038/s41570-018-0010-1 [65] HUANG L Z, WEI X, GAO E, et al. Single Fe atoms confined in two-dimensional MoS2 for sulfite activation: A biomimetic approach towards efficient radical generation[J]. Applied Catalysis B: Environmental,2019,268:118459. [66] CHEN P, GUO Y, NI J, et al. Efficient Ofloxacin degradation with Co(Ⅱ)-doped MoS2 nano-flowers as PMS activator under visible-light irradiation[J]. Chemical Engineering Journal,2020,401:125978. doi: 10.1016/j.cej.2020.125978 -





 下载:
下载: